Журнал «Почки» 3 (13) 2015
Вернуться к номеру
European Association of Urology 2015 Guidelines on Urological Infections
Авторы: Grabe M. (Chair), Bartoletti R., Bjerklund-Johansen T.E., Cai T. (Guidelines Associate), Çek M., Köves B. (Guidelines Associate), Naber K.G., Pickard R.S., Tenke P., Wagenlehner F., Wullt B.
Рубрики: Неврология
Разделы: Клинические исследования
Версия для печати
Статья опубликована на с. 47-58
Aim
The current Guidelines aim to provide both urologists and physicians from other medical specialities with evidence-based guidance regarding the treatment and prophylaxis of urinary tract infections (UTIs). These Guidelines cover male and female UTIs, male genital infections and special fields such as UTIs in paediatric urology and risk factors, e.g. immunosuppression, renal insufficiency and diabetes mellitus. Much attention is given to peri-operative antibacterial prophylaxis (ABP), aiming to reduce the overuse of antimicrobial agents in conjunction with surgery. High quality clinical research using strict internationally recognised definitions and classifications, as presented in these Guidelines, are encouraged.
3C.3. Acute episode of uncomplicated cystitis (lower UTI) in adults
3C.3.1. Diagnostic evaluation
3C.3.1.1. Clinical diagnosis
The diagnosis of acute uncomplicated cystitis can be made with a high probability based on a focused history of lower urinary tract symptoms (dysuria, frequency and urgency) and the absence of vaginal discharge or irritation, in those women who have no other risk factors for complicated UTIs [52, 63] (LE: 2a, GR: B). In elderly women genitourinary symptoms are not necessarily related to UTI [55].
In otherwise healthy diabetic patients with stable glycaemic metabolism, a sporadic or even recurrent cystitis can also be considered uncomplicated. However, in the long-term patients with diabetes may develop a neuropathic bladder with voiding disturbances which may be present as a re–levant complicating factor [57].
In otherwise healthy patients with mild and moderate renal insufficiency without other relevant structural and functional abnormalities within the urinary tract and the kidneys, a sporadic or recurrent cystitis can also be considered uncomplicated because no more serious outcome needs to be considered.
3C.3.1.2. Differential diagnosis
Symptomatic UTI should be differentiated from asymptomatic bacteriuria, which is considered not an infection but rather a commensal colonisation, which usually should not be treated and therefore not screened for, except if it is considered a risk factor in special situations (see Section 3B).
3C.3.1.3. Laboratory diagnosis
Urine dipstick testing, as opposed to urinary microscopy, is a reasonable alternative to culture for diagnosis of acute uncomplicated cystitis [64, 65] (LE: 2a, GR: B).
Urine cultures are recommended in the following situations:
— suspected acute pyelonephritis;
— symptoms that do not resolve or recur within 2–4 weeks after the completion of treatment;
— women who present with atypical symptoms [66, 67];
— pregnant women, and Males with suspected UTI (LE: 4, GR: B).
A colony count of > 103 cfu/mL of uropathogens is microbiologically diagnostic in women who present with symptoms of acute uncomplicated cystitis [68] (LE: 3, GR: B).
Women who present with atypical symptoms of either acute uncomplicated cystitis or acute uncomplicated pyelonephritis, as well as those who fail to respond to appropriate antimicrobial therapy should be considered for additional diagnostic studies (LE: 4, GR: B).
Urological evaluation including rectal examination should always be carried out in men to rule out relevant complicating factors (LE: 4,
GR: A).
3C.3.2. Disease management
Antibiotic therapy is recommended because clinical success is significantly more likely in women treated with antibiotics compared with placebo [69] (LE: 1a, GR: A). The choice of antibiotic therapy should be guided by [52]:
— spectrum and susceptibility patterns of the aetiological uropathogens;
— efficacy for the particular indication in clinical studies;
— tolerability and adverse reactions;
— adverse ecological effects;
— cost;
— availability.
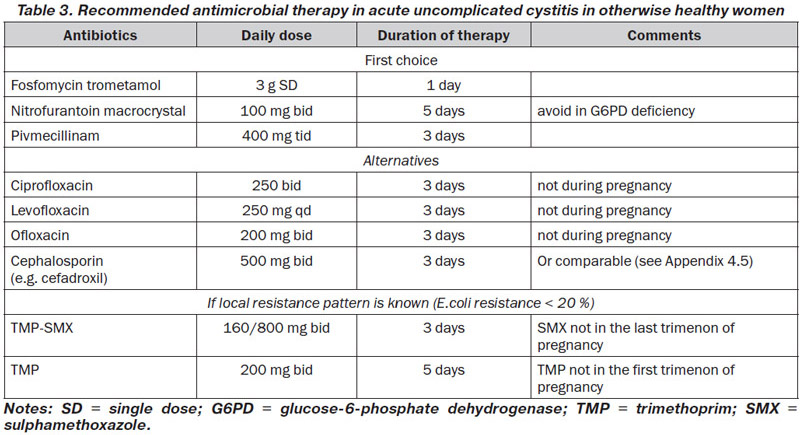
According to these principles and the available susceptibility patterns in Europe, fosfomycin trometamol 3 g single dose, pivmecillinam 400 mg tid for 3 days, and nitrofurantoin macrocrystal 100 mg bid for 5 days, are considered as drugs of first choice in many countries, when available [70–72] (LE: 1a, GR: A) (Table 3). These regimens are recommended for women, but not for men. Most ESBL-producing E.coli are still susceptible to fosfomycin. However, in Spain a parallel increase in community use of fosfomycin and resistance to fosfomycin in ESBL-producing E.coli has been observed [73].
Alternative antibiotics include trimethoprim alone or combined with a sulphonamide, and the fluoroquinolone class. Co-trimoxazole (160/800 mg bid for 3 days) or trimethoprim (200 mg for 5 days) should only be considered as drugs of first choice in areas with known resistance rates for E.coli of < 20 % [74, 75] (LE: 1b, GR: B). Despite still lower resistance rates in some areas, fluoroquinolones are not considered first choice because of adverse effects including negative ecological effects and selection of resistance (Table 3).
Aminopenicillins are no more suitable for empirical therapy because of the worldwide high E.coli resistance. Aminopenicillins in combination with a betalactamase inhibitor such as ampicillin/sulbactam or amoxicillin/slavulanic acid and oral cephalosporins are in general not so effective as short-term therapy and are not recommended for empirical therapy because of ecological collateral damage, but can be used in selected cases [76, 77].
Short courses of antimicrobial therapy can also be considered for the treatment of cystitis in pregnancy [78] (LE: 1a, GR: A), but not all antibiotics are suitable during pregnancy. In general penicillins, cephalosporins, fosfomycin, nitrofurantoin (not in case of G6P deficiency and during end of pregnancy), trimethoprim not in the first and sulphonamides not in the last trimenon, can be considered.
In men a treatment duration of at least 7 days is recommended, preferably with TMP-SMX or a fluoroquinolone if in accordance with the susceptibility testing (LE: 4; GR: B).
In patients with renal insufficiency the choice of antimicrobials may be influenced by the decreased renal excretion. Most antibiotics, however, have a wide therapeutic index. No adjustment of dose is necessary until GFR < 20 mL/min, except antibiotics with nephrotoxic potential, e.g. aminoglycosides. Combination of loop diuretics (e.g. furosemide) and a cephalosporin is nephrotoxic. Nitrofurantoin and tetracyclines are contraindicated, but not doxycycline.
3C.3.3. Follow-up
Routine post-treatment urinalysis or urine cultures in asymptomatic patients are not indicated [27] (LE: 2b, GR: B), except in pregnant women, if asymptomatic bacteriuria is an issue of therapy (see Chapter 3B.5.3). In women whose symptoms do not resolve by the end of treatment, and in those whose symptoms resolve but recur within 2 weeks, urine culture and antimicrobial susceptibility tests should be performed (LE: 4, GR: B). For therapy in this situation, one should assume that the infecting organism is not susceptible to the agent originally used. Retreatment with a 7-day regimen using another agent should be considered (LE: 4, GR: C).
3C.4.1. Diagnostic evaluation
3C.4.1.1. Clinical diagnosis
Acute pyelonephritis is suggested by flank pain, nausea and vomiting, fever (> 38 °C), or costovertebral angle tenderness, and it can occur in the absence of symptoms of cystitis [79].
Pregnant women with acute pyelonephritis need special attention, because this kind of infection may have not only an adverse effect on the mother with anaemia, renal and respiratory insufficiency, but also on the unborn with more frequent preterm labour and preterm birth [80].
Most men with febrile UTI have a concomitant infection of the prostate as measured by transient increases of PSA and prostate volume [81]. Thus, urological evaluation should be carried out routinely in men with febrile UTI, pyelonephritis, or recurrent UTI, or whenever a complicating factor is suspected (LE: 4, GR: A).
In diabetic patients with acute pyelonephritis metabolic abnormalities, e.g. hypo- and hyperglycaemia, hyperosmolar dehydration, or ketoacidosis, need to closely be followed [57]. Diabetic patients may also develop progression of renal parenchymal infection sometimes caused by gas-forming organisms, with a high mortality (emphysematous pyelonephritis), characterised histologically by acute pyogenic infiltration with micro-abscesses and the development of acute renal failure [82].
The origin of the organisms may be haematogenous. Intrarenal abscesses may rupture, leading to a perinephric collection and a psoas abscess, which occasionally may be indolent. Papillary necrosis is common in diabetics, particularly in association with acute pyelonephritis, resulting in renal parenchymal scarring, although it is difficult to exclude obstruction by the sloughed papillae as the cause of the nephropathy.
The risk of chronic renal disease and renal insufficiency caused by pyelonephritis is low. Underlying lesions including vesicoureteral reflux, analgesic abuse, nephrolithiasis and obstruction of the urinary tract have to be observed. However, acute bacterial infection, including pyelonephritis, can dramatically influence the progression of a chronic renal disease and vice versa chronic renal failure can alter the severity of an infection [58].
3C.4.1.2. Differential diagnosis.
It is most important to differentiate by appropriate imaging very early between an acute uncomplicated and complicated, mostly obstructive form of pyelonephritis, because the latter can very quickly lead to urosepsis.
3C.4.1.3. Laboratory diagnosis
Urinalysis (e.g. using a dipstick method), including the assessment of white and red blood cells and nitrites, is recommended for routine diagnosis [83] (LE: 4, GR: C). Colony counts > 104 cfu/mL of uropathogens are considered to be indicative of clinically relevant bacteriuria [84] (LE: 2b, GR: C).
3C.4.1.4. Imaging diagnosis
Evaluation of the upper urinary tract with ultrasound (US) should be performed to rule out urinary obstruction or renal stone disease (LE: 4, GR: C). Additional investigations, such as an unenhanced helical computed tomography (CT), excretory urography, or dimercaptosuccinic acid (DMSA) scanning, should be considered if the patient remains febrile after 72 h of treatment (LE: 4, GR: C). For diagnosis of complicating factors in pregnant women, US or magnetic resonance imaging (MRI) should be used preferentially to avoid radiation risk to the foetus (LE: 4, GR: B).
3C.4.2. Disease management
As a result of the lack of suitable surveillance studies, the spectrum and susceptibility patterns of uropathogens that cause uncomplicated cystitis can be used as a guide for empirical therapy [62] (LE: 4, GR: B). However, S.saprophyticus is less frequent in acute pyelonephritis as compared to acute cystitis (LE: 4, GR: B).
3C.4.2.1. Mild and moderate cases
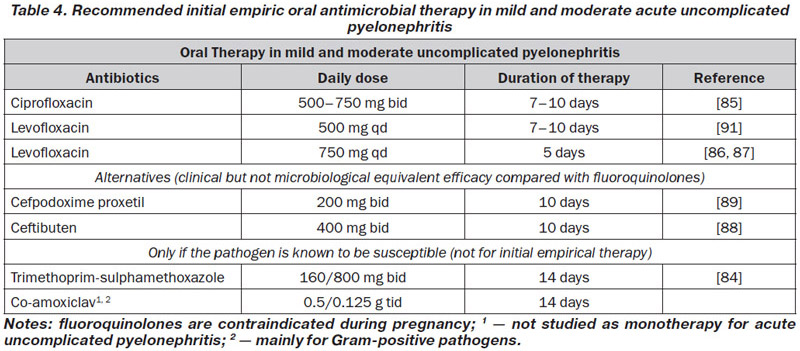
In mild and moderate cases of acute uncomplicated pyelonephritis (see Table 4), oral therapy of 10–14 days is usually sufficient (LE: 1b, GR: B). A fluoroquinolone for 7–10 days can be recommended as first-line therapy if the resistance rate of E.coli is still < 10 % [85] (LE: 1b, GR: A).
If the fluoroquinolone dose is increased, the treatment can probably be reduced to 5 days [86, 87] (LE: 1b, GR: B). However, increasing numbers of fluoroquinolone-resistant E.coli in the community have already been found in some parts of the world, thus restricting the empirical use of fluoroquinolones, and fluoroquinolones are contraindicated during pregnancy.
A third-generation oral cephalosporin, such as cefpodoxime proxetil or ceftibuten, could be an alternative [88, 89] (LE: 1b, GR: B). However, available studies have demonstrated only equivalent clinical, but not microbiological, efficacy compared with ciprofloxacin.
As a result of increasing E. coli resistance rates > 10 %, cotrimoxazole is not suitable for empirical therapy in most areas, but it can be used after sensitivity has been confirmed through susceptibility testing [90] (LE: 1b, GR: B).
Co-amoxiclav is not recommended as a drug of first choice for empirical oral therapy of acute pyelonephritis (LE: 4, GR: B). It is recommended when susceptibility testing shows a susceptible Gram-positive organism (LE: 4, GR: C).
In communities with high rates of fluoroquinolone-resistant and ESBL-producing E.coli (> 10 %), initial empirical therapy with an aminoglycoside or carbapenem has to be considered until susceptibility testing demonstrates that oral drugs can also be used (LE: 4, GR: B).
3C.4.2.2. Severe cases
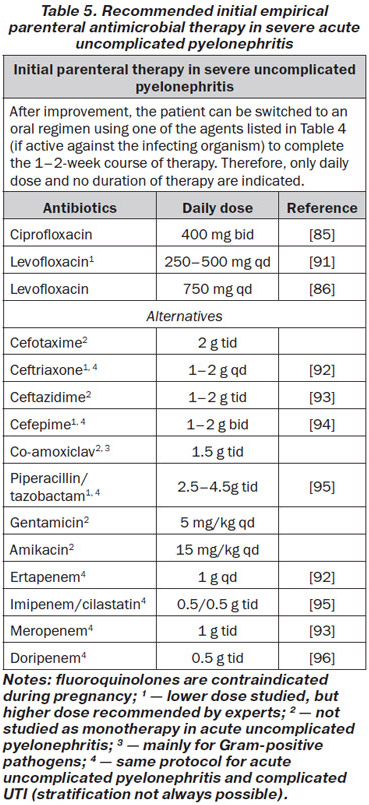
Patients with severe pyelonephritis who cannot take oral medication because of systemic symptoms such as nausea and vomiting, have to be treated initially with one of the following parenteral antibiotics (Table 5).
Hospital admission should be considered if complicating factors cannot be ruled out by available diagnostic procedures and/or the patient has clinical signs and symptoms of sepsis (LE: 4, GR: B).
After improvement, the patient can be switched to an oral regimen using one of the antibacterials mentioned in Table 4, if active against the infecting organism, to complete the 1–2-week course of therapy (LE: 1b, GR: B).
In pregnant women with pyelonephritis outpatient management with appropriate antibiotics may also be considered, provided symptoms are mild and close follow-up is feasible [97, 98] (LE: 1b, GR: A).
In more severe cases of pyelonephritis, hospitalisation and supportive care are usually required. After clinical improvement parenteral therapy can also be switched to oral therapy for a total treatment duration of 7–10 days (LE: 4,GR: B).
In men with febrile UTI, pyelonephritis, or recurrent infection, or whenever a complicating factor is suspected a minimum treatment duration of 2 weeks is recommended preferably with a fluoroquinolone since prostatic involvement is frequent [99] (LE: 2a, GR: B).
3C.4.3. Follow-up
Routine post-treatment urinalysis and urine cultures in an asymptomatic patient might not be indicated (LE: 4, GR: C), except in pregnant women, if asymptomatic bacteriuria is a treatment issue see Section 3B.5.3.
In patients whose pyelonephritis symptoms do not improve within 3 days, or resolve and then recur within 2 weeks, repeated urine culture and antimicrobial susceptibility tests and an appropriate investigation, such as renal US, CT or renal scintigraphy, should be performed (LE: 4, GR: B).
In patients with no urological abnormality, it should be assumed that the infecting organism is not susceptible to the agent originally used, and an alternative tailored treatment should be considered based on culture results (LE: 4, GR: B).
For patients who relapse with the same pathogen, the diagnosis of uncomplicated pyelonephritis should be reconsidered. Appropriate diagnostic steps are necessary to rule out any complicating factors (LE: 4, GR: C).
3C.5. Recurrent uncomplicated UTIs in adult women
3C.5.1. Diagnostic evaluation
Recurrent UTIs are common among young, healthy women, even though they generally have anatomically and physiologically normal urinary tracts [100] (LE: 2a). Common risk factors are given in Table 2.
Recurrent UTIs need to be diagnosed by urine culture (LE: 4, GR: A). Imaging of the upper urinary tract and cystoscopy are not routinely recommended for evaluation of women with recurrent UTIs [101] (LE: 1b, GR: B) but should be performed without delay in atypical cases. Also, residual urine should be excluded (LE: 4, GR: B).
Recurrent UTIs in men are not included here because this may be a sign of exacerbation from chronic bacterial prostatitis (see Chapter 3I). Also not included here are recurrent UTI due to complicating urological factors, such as urinary catheters, nephrolithiasis and neuropathic bladder voiding disturbances, among others.
3C.5.2. Disease management and follow-up
Prevention of rUTI includes i) counselling and behavioural modifications, i.e. avoidance of risk factors, ii) non-antimicrobial measures and iii) antimicrobial prophylaxis, which should be attempted also in this order. Urological risk factors need to be looked for and eliminated as far as possible. Significant residual urine should be treated optimally, which also includes clean intermittent catheterisation (CIC) when valued necessary.
3C.5.2.1. Risk factors and behavioural modifications
A number of measures such as fluid intake and personal hygiene behaviours (e.g. reduced fluid intake, habitual and post-coitial delayed urination, wiping from back to front after defection, douching and wearing occlusive underwear) have been suggested to increase the risk of UTI. However, studies that have explored these risk factors have consistently documented the lack of association with recurrent UTI.
In young healthy women, sexual intercourse is the risk factor most highly associated with rUTI. Others include spermicide use, having a new sex partner, having a mother with history of UTI, and having UTI during childhood.
The most common risk factors in postmenopausal women are given in Table 2. There is growing evidence that UTIs in children and adults are associated with genetic mutations that affect the innate immune system [54].
3C.5.2.2. Non-antimicrobial prophylaxis
There are many non-antimicrobial measures recommended for recurrent UTI but only a few result from well- designed studies and are therefore able to make evidence-based recommendations [102, 103].
Hormonal replacement
In postmenopausal women local, vaginal oestrogen replacement, but not oral oestrogen, showed a trend towards preventing UTI recurrences, but vaginal irritation occurred in 6–20 % of women [103, 104] (LE: 1b, GR: C).
Immunoactive prophylaxis
OM-89 (Uro-Vaxom®) is sufficiently well documented and has been shown to be more effective than placebo in several randomised trials with a good safety profile. Therefore, it can be recommended for immunoprophylaxis in female patients with recurrent uncomplicated UTI [103, 105, 106] (LE: 1a, GR: B). Efficacy in other groups of patients and relative to antimicrobial prophylaxis remains to be established.
The vaginal vaccine Urovac® slightly reduced UTI recurrence and primary immunisation followed by booster immunisation increased time to re-infection [103] (LE: 1a, GR: C).
For parenteral immunotherapeutic products on the market, larger phase III studies are still missing. In smaller phase II studies, StroVac® and Solco-Urovac® have been shown to be effective when administered with a booster cycle of the same agents (LE: 1a, GR: C).
For other immunotherapeutic products, no controlled studies are available. Therefore, no recommendations are possible.
Prophylaxis with probiotics (Lactobacillus sp)
Accessibility of clinically proven probiotics for UTI prophylaxis is currently not universal. Only the Lactobacillus strains specifically tested in studies should be considered for prophylaxis.
When commercially available, it is reasonable to consider the use of intravaginal probiotics that contain L.rhamnosus GR-1 and L.reuteri RC-14 for the prevention of recurrent UTI [107], and these products can be used once or twice weekly (LE: 4, GR: C). Vaginal application of Lactobacillus crispatus reduced the rate of recurrent UTI in pre-menopausal women in one study, and can also be used if available [108] (LE: 1b, GR: B).
Daily use of the oral product with strains GR-1 and RC-14 is worth testing given that it can restore the vaginal lactobacilli, compete with urogenital pathogens, and prevent bacterial vaginosis, a condition that increases the risk of UTI [102]. However, oral lactobacilli prophylaxis did not decrease UTI recurrence [103], therefore no recommendations are possible.
In summary, pooled data from meta-analyses of available RCTs show no convincing benefit of Lactobacillus products as prophylaxis of recurrent UTI. However differences in effectiveness between available preparations suggest further trials are needed before any recommendation for use can be made.
Recommendation: Do not use outside of investigational trials.
Prophylaxis with cranberry
Previous limited studies have suggested that cranberry (Vaccinium macrocarpon) is useful in reducing the rate of lower UTIs in women [109, 110]. A recent meta-analysis including 24 studies and comprising 4,473 participants showed however that cranberry products did not significantly reduce the occurrence of symptomatic UTI overall or for any of the following sub-groups: children with recurrent UTIs, older people, women with recurrent UTIs, pregnant women, cancer patients, or people with neuropathic bladder or spinal injury [111]. Due to these contradictory results, no recommendation of the daily consumption of cranberry products can be made.
Prophylaxis with d-mannose
In a recent randomised placebo-controlled non-blinded clinical trial, it was shown that a daily dose of 2g d-mannose was significantly superior to placebo and as effective as 50 mg nitrofurantoin in preventing recurrent UTI [112]. This is indicative but not sufficient for a recommendation. D-mannose should at the present time only be used within the frame of high quality clinical investigations.
Endovesical instillation
Endovesical instillation of hyaluronic acid and chondroitin sulphate have been used for glycosaminoglycan (GAG) layer replenishment in the therapy of interstitial cystitis, overactive bladder, radiation cystitis, and for prevention of recurrent UTI. A recent review of 27 clinical studies concluded that large-scale trials are urgently needed to underline the benefit of this type of therapy [113]. Therefore, no general recommendation is possible at this stage.
3C.5.2.3. Antimicrobial prophylaxis
Antimicrobial prophylaxis can be given continuously (daily, weekly) for longer periods of time (3–6 months), or as a single post-coital dose. Continuous or post-coital antimicrobial prophylaxis [114] for prevention of recurrent UTI should be considered only after counselling and behavioural modification has been attempted, and when non-antimicrobial measures have been unsuccessful (LE: 4, GR: B).
In appropriate women with recurrent uncomplicated cystitis, self-diagnosis and self-treatment with a short course regimen of an antimicrobial agent should be considered [115] (LE: 2b, GR: A). The choice of antibiotics is the same as for sporadic acute uncomplicated UTI (Table 3).
Postcoital prophylaxis should be considered in pregnant women with a history of frequent UTIs before onset of pregnancy, to reduce their risk of UTI [116] (LE: 2b, GR: B).
Continuous antimicrobial prophylaxis regimens for women with recurrent UTIs include e.g. nitrofurantoin (macrocrystal) 50 mg or 100 mg once daily, fosfomycin trometamol 3 g every 10 days, and during pregnancy e.g. cephalexin 125 mg or 250 mg or cefaclor 250 mg once daily [100].
In general, the choice of antibiotics should be based upon the identification and susceptibility pattern of the organism causing the UTI, the patient’s history of drug allergies and the ecological collateral effects including bacterial selection of resistance by the chosen antimicrobial. Using these principles, several issues need to be considered:
— Ecological collateral effects mean that oral fluoroquinolones and cephalosporins are no longer recommended routinely, except in specific clinical situations.
— The worldwide increase of E.coli resistance against trimethoprim casts doubts on trimethoprim with or without a sulphonamide to be an effective prophylactic agent still.
— There are recent warnings by governmental agencies for the long-term prophylactic use of nitrofurantoin because of the rare but severe pulmonary and hepatic adverse effects [117].
Altogether this underlines the need for reconsidering long-term antibiotic prophylaxis in recurrent UTI and assess in each individual case effective alternative preventive measures.