Журнал «Боль. Суставы. Позвоночник» Том 7, №4, 2017
Мінеральна щільність кісткової тканини, показники T та Z у молодих чоловіків з ювенільним ідіопатичним артритом
Резюме
Актуальність. Ювенільний ідіопатичний артрит (ЮІА) — це термін, що використовується для позначення групи гетерогенних педіатричних ревматичних захворювань. Багато з цих станів персистують і в більш зрілому віці. Наявність хронічного запального захворювання разом із лікуванням глюкокортикоїдами є фактором ризику остеопорозу в молодих чоловіків. Метою роботи було вивчення мінеральної щільності кісткової тканини (МЩКТ), показників T і Z у молодих чоловіків з ЮІА. Матеріали та методи. Дослідження включало 50 пацієнтів віком 19–25 років, розподілених на дві групи: I — 25 практично здорових молодих чоловіків; ІІ — 25 молодих чоловіків із різних регіонів України, у яких у дитинстві діагностований ЮІА, незалежно від наявності або відсутності активного запалення під час спостереження. Двохенергетична рентгенівська денситометрія (Prodigy, GE Lunar, Madison, США) була проведена в Інституті геронтології ім. Д.Ф. Чеботарьова, Українському науково-медичному центрі проблем остеопорозу разом з аналізом МЩКТ, показників T і Z у різних частинах скелета. Результати. Молоді пацієнти з ЮІА та здорові чоловіки не відрізнялися за віком, ростом, вагою й індексом маси тіла. У 4 пацієнтів з ЮІА (16 %) були переломи, тоді як у контрольній групі їх не відзначалося. Негативний вплив ЮІА на МЩКТ було виявлено в II групі на відміну від першої. МЩКТ поперекового відділу хребта в II групі була нижчою (p < 0,01), ніж у здорових суб’єктів, як і показник Z (p < 0,001) на рівні L1–L4 поперекового відділу хребта. МЩКТ, індекси T і Z в ділянці шийки стегнової кістки були нижчими в II групі, ніж у першій (p < 0,001; p < 0,001; p < 0,01 відповідно). Значні відмінності між двома групами були виявлені щодо загальної МЩКТ (p < 0,001), показників T (p < 0,01) і Z (p < 0,05). У пацієнтів з ЮІА нижчими були МЩКТ (p < 0,01) й індекс T (p < 0,05) в ультрадистальній ділянці променевої кістки. Зниження МЩКТ до рівня остеопенії (показник Z < –2 SD) було виявлено у 20 % пацієнтів на рівні L1–L4 поперекового відділу хребта, у 8 % — в ділянці шийки стегнової кістки, у 12 % — всього організму і у 8 % пацієнтів — в ультрадистальній ділянці променевої кістки. Висновки. У молодих чоловіків з ЮІА віком 19–25 років відзначається зниження загальної МЩКТ, показників T і Z, що вказує на негативний вплив захворювання на кісткову тканину порівняно зі здоровими чоловіками відповідного віку.
Актуальность. Ювенильный идиопатический артрит (ЮИА) — это термин, используемый для обозначения группы гетерогенных педиатрических ревматических заболеваний. Многие из этих состояний персистируют и в более зрелом возрасте. Наличие хронического воспалительного заболевания наряду с лечением глюкокортикоидами является фактором риска остеопороза у молодых мужчин. Целью работы было изучение минеральной плотности костной ткани (МПКТ), показателей T и Z у молодых мужчин с ЮИА. Материалы и методы. Исследование включало 50 пациентов в возрасте 19–25 лет, разделенных на две группы: I — 25 практически здоровых молодых мужчин; ІІ — 25 молодых мужчин из разных регионов Украины, у которых в детстве диагностирован ЮИА, независимо от наличия или отсутствия активного воспаления во время наблюдения. Двухэнергетическая рентгеновская денситометрия (Prodigy, GE Lunar, Madison, США) была проведена в Институте геронтологии им. Д.Ф. Чеботарева, Украинском научно-медицинском центрe проблем остеопороза наряду с анализом МПКТ, показателей T и Z в различных частях скелета. Результаты. Молодые пациенты с ЮИА и здоровые мужчины не отличались по возрасту, росту, весу и индексу массы тела. У 4 пациентов с ЮИА (16 %) были переломы, тогда как в контрольной группе их не отмечалось. Отрицательное воздействие ЮИА на МПКТ было обнаружено во II группе в отличие от первой. МПКТ поясничного отдела позвоночника во II группе была ниже (p < 0,01), чем у здоровых субъектов, как и показатель Z (p < 0,001) на уровне L1–L4 поясничного отдела позвоночника. МПКТ, индексы T и Z в области шейки бедренной кости были ниже во II группе, чем в первой (p < 0,001; p < 0,001; p < 0,01 соответственно). Значительные различия между двумя группами были обнаружены в отношении общей МПКТ (p < 0,001), показателей T (p < 0,01) и Z (p < 0,05). У пациентов с ЮИА более низкими были МПКТ (p < 0,01) и индекс T (p < 0,05) в ультрадистальной области лучевой кости. Снижение МПКТ до уровня остеопении (показатель Z < –2 SD) было обнаружено у 20 % пациентов на уровне L1–L4 поясничного отдела позвоночника, у 8 % — в области шейки бедренной кости, у 12 % — всего организма и у 8 % пациентов — в ультрадистальной области лучевой кости. Выводы. У молодых мужчин с ЮИА в возрасте 19–25 лет отмечается снижение общей МПКТ, показателей T и Z, что указывает на отрицательное воздействие заболевания на костную ткань по сравнению со здоровыми мужчинами соответствующего возраста.
Background. Juvenile Idiopathic Arthritis (JIA) is a term used to classify a group of heterogeneous pediatric rheumatic diseases. Many of these conditions persist through adulthood. Presence of chronic inflammatory disease along with a glucocorticoid treatment is the risk factor of osteoporosis in young adult males. The purpose was to study the bone mineral density (BMD), T- and Z-scores in young adult males with JIA. Materials and methods. The study included 50 patients aged 19–25 years, divided into two groups: I — 25 apparently healthy young males; ІІ — 25 young men from different regions of Ukraine with a history of JIA in childhood, regardless of the presence or absence of active inflammation at the time of the observation. Two-energy X-ray densitometry (Prodigy, GE Lunar, Madison, USA) was performed at the D.F. Chebotarev Institute of Gerontology, Ukrainian Scientific-Medical Centre for the Problems of Osteoporosis, together with analysis of BMD, T- and Z-scores at different skeletal areas. Results. Young men with JIA and healthy individuals did not differ in age, height, weight and body mass index. Four patients with JIA (16 %) had fractures, while in the control group, there were no fractures. Negative impact of the JIA on the BMD was found in group II compared to group I. Lumbar spine BMD in group II was lower (p < 0.01) than in healthy subjects, as well as the Z-score (p < 0.001) in the L1-L4 lumbar spine region. BMD, T- and Z-scores in femoral neck region were lower in group II than in group I (p < 0.001; p < 0.001; p < 0.01, respectively). Significant differences between the two groups were found in total body BMD (p < 0.001), T-score (p < 0.01), Z-score (p < 0.05). Patients with JIA had lower BMD (p < 0.01) and T-score (p < 0.05) of the ultradistal radius. Decrease of BMD up to the level of osteopenia (Z-score < –2 SD) was found in 20 % patients at the level of L1-L4 lumbar spine, in 8 % — at femoral neck, in 12 % — at total body and in 8 % patients at the level of ultradistal radius. Conclusions. Young men with JIA aged 19–25 years had reduced total body BMD, T- and Z-scores, which indicate the negative impact of the disease on the bone tissue compared with healthy men of the corresponding age.
Ключевые слова
ювенільний ідіопатичний артрит; мінеральна щільність кісткової тканини; молоді чоловіки; остеопороз; остеопенія; показник Т; показник Z
ювенильный идиопатический артрит; минеральная плотность костной ткани; молодые мужчины; остеопороз; остеопения; показатель Т; показатель Z
juvenile idiopathic arthritis; bone mineral density; young males; osteoporosis; osteopenia; T-score; Z-score
Introduction
Identifying the factors that affect the development of osteoporosis (OP) associated with low-energy fractures in women and young men is important not only for medical and preventive care, but also for society as a whole. Risk factors of early secondary OP include administration of some drugs (including glucocorticoids) and presence of chronic inflammatory diseases (endocrine, autoimmune, including systemic connective tissue diseases). It is important to study bone mineral density (BMD) in adults who have been diagnosed with inflammatory joint lesions in childhood — juvenile idiopathic arthritis. This disease includes different forms of arthritis of unknown etio–logy (other than rheumatoid arthritis), begins at the age under 16, often continues in adulthood [1] and can affect the formation of BMD, due to a prolonged administration of glucocorticoids and chronic systemic inflammation. In previous studies, we examined BMD in women with JIA, where it was shown that the presence of JIA in childhood affects the formation of peak bone mass in female and leads to a decrease in BMD in adults requiring active monitoring and preventive therapy in childhood and, if necessary, adult treatment for prevention of osteoporotic complications [2, 3]. Diagnosis and treatment of OP in young people aged 20–45 years remain insufficiently stu–died. There are difficulties in the differentiation of young healthy people with low bone density that reflects low peak bone mass due to their mass and height, puberty period, hereditary factors and environmental impact during growth [4–6]. Differential diagnosis may be complicated by the fact that 30 % of young women and 50 % of young men usually have had traumatic fractures in childhood and adolescence [7–11]. These fractures are associated with a decrease in bone mass and decrease in the formation of peak bone mass in healthy individuals [12]. It is known that the annual frequency of vertebral fractures in young patients (< 35 years) is 3 cases per 100.000 persons, with an increase of up to 21 cases in people aged 35–44 years, often associated with a trauma [13]. Difficulties in diagnosis of OP in young people appear due to several problems: 1) low bone mass and/or 2) presence of peripheral fractures which are not necessarily related to the fragility of the skeleton. It is known that OP develops less often in males than in females due to higher peak bone mass, larger diameter of long bones and lower rates of bone loss in men. The maximum frequency of osteoporotic fractures in men is registered 10 years later than in women, and the overall risk of osteoporotic fractures at age 50 is 39.7 % for women and 13.1 % for men [14]. In 2012, the Endocrine Society published the Clinical Practice Guidelines, which highlights the risk of OP developing in men [15]. According to these Guidelines, the following factors affect the male BMD: genetic and constitutional factors, here–dity (low energy fractures suffered by the parents), low body mass index (< 20 kg/m2), a large hip axial length, lifestyle and dietary characteristics (insufficient calcium and vitamin D intake, smoking, alcohol abuse, low physical activity, prolonged immobilization), certain diseases (endocrine diseases, blood diseases, rheumatic diseases, chronic obstructive pulmonary diseases, neurological diseases, organ transplantation, hypogonadism) and administration of certain drugs (glucocorticoids, thyroid drugs, anticoagulants, gonadotrophin releasing hormone agonists, anticonvulsants). In this light, it is interesting to study the bone status in young men with JIA.
Purpose: to study the bone mineral density, T- and Z-scores in young adult males with JIA.
Materials and methods
The study included 50 adult men aged 19–25 years, divided into two groups: I — 25 practically healthy young males; ІІ — 25 young men with a history of JIA in childhood regardless of the presence or absence of active inflammation at the time of the observation. The second group included patients from different regions of Ukraine who have been diagnosed with the JIA during the period between 1984 and 2013. The inclusion criteria included patients with a diagnosis of JIA according to the classification criteria of ILAR, Durban 1997, Edmonton 2001 (Petty R.E. et al., 2004) [16]. The retrospective analysis of patients’ medical records was made, the age of the disease onset, delay in diagnosis and received medical treatment were analyzed. In adulthood, all patients with JIA were examined by an adult rheumatologist on the basis of the Oleksandrivska City Clinical Hospital in Kyiv, Ukraine, between April 2015 and February 2017. Demographic and anthropometric data, duration of the disease, ILAR-variant in the onset of the disease were analyzed. All patients underwent dual-energy X-ray absorptiometry at the SI “D.F. Chebotarev Institute of Gerontology NAMS of Ukraine”, Ukrainian Scientific-Medical Centre for the Problems of Osteoporosis. Dual-energy X-ray absorptiometry (Prodigy, GE Lunar, Madison, USA) was performed with analysis of BMD, T- and Z-scores at different skeletal areas.
Statistical analysis was performed using descriptive statistics, Student’s criteria for unrelated variables, and one-way ANOVA dispersion analysis. We also used the software package Statistica 6.0 by Copyright StatSoft, Inc. 1984–2001.
Results
The analysis of anthropometric data and bone tissue characteristics of young men with JIA and healthy individuals matched for age and sex, were carried out (table 1).
/11-1.jpg)
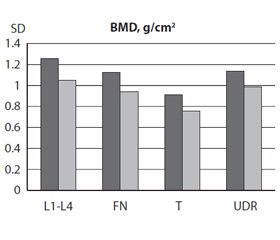
The I and II groups did not differ in age, height, weight and BMI. Low-energy fractures were found in 4 patients (16 %) in the the JIA group, while there were no patients with fractures in the control group. A negative impact of the JIA on the BMD was found in the II group compared to the I group. The lumbar spine (L1–L4) BMD in the II group (1.052 ± 0.036 g/cm2) was lower (p < 0.01) than in the I group (1.255 ± 0.041 g/cm2), as well as the Z-score (p < 0.001) at the lumbar spine level. Femoral neck BMD in the II group was 0.942 ± 0.039 g/cm2 versus 1,128 ± 0.025 g/cm2 in the I group (p < 0.001), the T-score in this area in the II group was –0.98 ± 0.32 versus 0.46 ± 0.18 in healthy patients (p < 0.001), Z-score –0.64 ± 0.27 in the II group versus 0.33 ± 0.20 in the I group (p < 0.01). Significant differences between the two groups were found in total body BMD (p < 0,001), T-score (p < 0.01) and Z-score (p < 0.05). The ultradistal radius BMD in the II group was 0.987 ± 0.043 g/cm2 compared to the control group 1.136 ± 0.028 g/cm2 (p < 0.01), T-score (p < 0.05). Figure 1 shows the BMD, T- and Z-scores at different sites of the skeleton of young men with JIA and healthy individuals.
/12-1.jpg)
It is known that OP is diagnosed when the BMD is less than or equal to 2.5 standard deviations below that of a young (20–40 year-old), healthy adult women reference population (T-score ≤ –2.5 SD) [17, 18]. However, low bone mass in children and adolescents is determined by BMD decrease by more than –2 SD compared to practically healthy people of the same age (Z-score < –2 SD) [19] and it is recommended to diagnose bone fragility not only on the basis of low bone density, but also due to presence of low-energy fractures [20]. BMD’s decrease to a level of osteopenia (Z-score below –2 SD) was found in 5 (20 %) patients at L1–L4, in 2 (8 %) patients at femoral neck, in 3 (12 %) patients at total body and in 2 (8 %) patients at ultradistal radius. According to the study made in 2012, osteoporosis of spine and femoral neck is diagnosed in young people with diseases that affect the bone tissue metabolism when T is < –2.5 SD, similar to postmenopausal women and men older than 50 years [6]. In our study, osteoporosis according to T-score was detected in 4 (16 %) patients at lumbar spine (L1–L4), in 4 (16 %) patients at the level of femoral neck, 6 (24 %) patients at the level of total body and 2 (8 %) patients at ultra distal radius.
Discussion
It has been shown that young men with JIA aged 19–25 have reduced BMD at all areas of the skeleton, indicating the negative effect of the disease on the bone tissue in this category of patients compared to healthy men of the corresponding age. Unlike healthy people, whose peak bone mass is achieved in adolescence, patients with JIA had peak bone mass formation suppressed by direct and indirect mechanisms, namely due to the presence of inflammatory disease, drug therapy and immobilization.
In our study we did not take into account disease acti–vity and its association with the BMD changes based on the results of V. Vasdev et al. [21] where it was shown that OP develops in young men with early ankylosing spondylitis, which is a chronic inflammatory disease like JIA. BMD does not depend on the activity of the disease and its total duration. According to the results of studies of the structu–ral and functional state of bone tissue, OP was found in 1 % of men aged 20–29 years in Ukraine [22], however, in our study OP in patients with JIA was detected in 8–24 % of patients at different sites of the skeleton, which is significantly higher than in general population. Comparing similar data studies, osteopenic BMD reductions by the Z-score and T-score at the levels of L1–L4, femoral neck, and ultradistal radius were found more frequently in young women with JIA [2], while osteopenia by T-score in total body was found in 24 % of observed men versus 15 % in women, accor–ding to our previous studies [3]. On the one hand, this diffe–rence may be due to a small group of male patients that are included in the study. On the other hand, the study of the National Health and Nutrition USA (NHANES) III, based on the results of the largest single studies of femoral neck BMD in adult men [23–26] proves that the peak of BMD at hip and lumbar spine is reached at the age of 20–30 years, while BMD peak of total body and ultradistal radius are reached in men after 30 years. This may explain why our group of patients who haven’t reached the peak BMD yet, had higher rates of osteopenia. Therefore, our results should be interpreted with caution because the study included patients aged under 30, who may not have reached the peak BMD at the affected areas.
Our research has some limitations. First, it was a cross-sectional study, which limits the assessment of the natural course of JIA and prognostic factors of OP in the JIA. Se–condly, the influence of drugs, including glucocorticoids and biological drugs, on the BMD was not taken into account. Previous studies showed that treatment, geographical origin and socio-economic differences may have effects on BMD at JIA.
Nevertheless, our study also has its strengths: this is the first study in Ukraine where BMD is studied in young adult males with JIA aged 19–25 years, and it is a controlled study with control group matching by age and gender.
Conclusion
Young men with JIA aged 19–25 years have a reduced total body BMD, T-, Z-score, which indicates the negative impact of the disease on the bone tissue compared to healthy men of the corresponding age.
Conflict of interest. The authors declare having no conflicts of interest that may be perceived as being likely to prejudice the impartiality of the article.
Sources of funding. This article has not received financial support from government, public or commercial organizations.
Список литературы
1. Bertilsson L, Anderson-Gare B, Fasth A, Petersson IF, Forsblad-D’elia H. Disease course, outcome and predictors of outcome in a population-based juvenile chronic arthritis cohort followed for 17 years. J Rheumatol. 2013;40(5):
715-724. doi.org/10.3899/jrheum.1206022.
2. Povoroznyuk VV, Dzhus MB. Mineral bone density in young women with juvenile idiopathic arthritis. Bol’, Sustavy, Pozvonochnik. 2017;7(2):41-49. doi: 10.22141/2224-1507.7.2.2017.108696. (in Ukrainian).
3. Povoroznyuk VV, Amosova KM, Dzhus MB. Age pe–cularities of bone mineral density in young women with juvenile idiopathic arthritis. Ukr Rheum J. 2017;3:22-38.
4. Bonjour JP, Chevalley T, Rizzoli R, Ferrari S. Gene-environment interactions in the skeletal response to nutrition and exercise during growth. Med Sport Sci. 2007;51:64-80. doi: 10.1159/000103005.
5. Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res. 2006;9(21):1489-1495. doi.org/10.1359/jbmr.0601.
6. Ferrari S, Bianchi ML., Eisman JA, Foldes AJ, Adami S, Wahl DA, Stepan JJ, de Vernejoul M-C, Kaufman J-M. Osteoporosis in young adults: pathophysiology, diagnosis, and management. Osteoporos Int. 2012;23:2735-2748. doi: 10.1007/s00198-012-2030-x.
7. Bailey DA, Wedge JH, McCulloch RG, Martin AD, Bernhardson SC. Epidemiology of fractures of the distal end of the radius in children as associated with growth. J Bone Joint Surg Am. 1989;8(71-A):1225-1231. doi: 10.2106/00004623-198971080-00016.
8. Haugen M, Lien G, Flatø B, et al. Young adults with juvenile arthritis in remission attain normal peak bone mass at the lumbar spine and forearm. Arth.&Rheum. 2000;43(7):1504-1510. doi: 10.1002/1529-0131(200007)43:7<1504::AID-ANR13>3.0.CO;2-0.
9. Lien G, Flatø B, Haugen M, et al. Frequency of osteopenia in adolescents with early-onset juvenile idiopathic arthritis: a long-term outcome study of one hundred five patients. Arth.&Rheum. 2003;48(8):2214-2223. doi:
10.1002/art.11097.
10. Pepmueller PH, Cassidy JT, Allen SH, Hillman LS. Bone mineralization and bone mineral metabolism in children with juvenile rheumatoid arthritis. Arth.&Rheum. 1996;39(5):746-757. doi: 10.1002/art.1780390506.
11. Seeman E, Bianchi G, Khosla S, Kanis JA, Orwoll E. Bone fragility in men — where are we? Osteoporos Int. 2006;17(11):1577-1583. doi: 10.1007/s00198-006-0160-8.
12. Ferrari SL, Chevalley T, Bonjour JP, Rizzoli R. Childhood fractures are associated with decreased bone mass gain during puberty: an early marker of persistent bone fragility? J Bone Miner Res. 2006;21(4):501-507. doi: 10.1359/jbmr.051215.
13. Curtis EM, Moon RJ, Harvey NC, Cooper C.The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Intern J of Orthopaed and Trauma Nursing. 2017;26:7-17. http://dx.doi.org/10.1016/j.bone.2017.01.024.
14. Adler RA. Osteoporosis in men: insights for the clinician. Ther Adv Musculoskelet Dis. 2011;3(4):191-200. doi: 10.1177/1759720X11411600.
15. Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in Men: An Endocrine Society Clinical Practice Guideline. J of Clin Endocrin&Metabol. 2012;97(6):1802-1822. https://doi.org/10.1210/jc.2011-3045.
16. Petty RE, Southwood TR, Manners P, et al, International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton 2001. J Rheumatol. 2004;31:390-2. PMID: 14760812.
17. Riis BJ. Biochemical markers of bone turnover II: Diagnosis, prophylaxis, and treatment of osteoporosis. American Journal of Medicine. 1993;5(S1):S17-S21.
doi: 10.1016/0002-9343(93)90376-Z.
18. Kanis JA, Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Sy–nopsis of a WHO report. Osteoporosis International. 1994; 4(6):368-381. PMID: 7696835.
19. Bianchi ML. Osteoporosis in children and adolescents. Bone. 2007;4(41):486-495. doi.org/10.1016/j.bone.2007.07.008.
20. Baim S, Binkley N, Bilezikian JP, Kendler DL, et al. Official Positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Position Development Conference. J Clin Densitom. 2008;11:75-91. doi.org/10.1016/j.jocd.2007.12.007.
21. Vasdev V, Bhakuni D, Garg MK, Narayanan K, Jain R, Chadha D. Bone mineral density in young males with ankylosing spondylitis. Int J Rheum Dis. 2011;14(1):68-73. doi: 10.1111/j.1756-185X.2010.01577.x. Epub 2010
Nov 9.
22. Povoroznyuk VV, Orlik TV, Kreslov EO. Contemporary approach to the problem of osteoporosis in men in Ukraine. Bol’, Sustavy, Pozvonochnik. 2012;2(06):6-9.
23. Bonny L Specker, Howard E Wey, Eric P Smith. Rates of bone loss in young adult males. Int. J Clin Rheumtol. 2010;5(2):215-228. doi: 10.2217/ijr.10.7.
24. Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468-490.
25. Marwaha RK, Tandon N, Shivapasad C, et al. Peak bone mineral density of physically active healthy Indian men with adequate nutrition and no known current constraints to bone mineralization. J Clin Densitom. 2009;12:314-321. doi: 10.1016/j.jocd.2009.05.004.
26. Henry MJ, Pasco JA, Korn S, et al. Bone mineral density reference ranges for Australian men: Geelong Osteoporosis Study. Osteoporos Int. 2010;21(6):909-17. doi: 10.1007/s00198-009-1042-1047.

/11-1.jpg)
/12-1.jpg)