Артеріальна гіпертензія (АГ) є основним фактором ризику серцево-судинних та ниркових захворювань у Сполучених Штатах та у всьому світі. Ожиріння є одним з найбільших факторів ризику розвитку первинної гіпертензії через декілька механізмів, включаючи нейрогормональну активацію, запалення та дисфункцію нирок. Оскільки поширеність ожиріння продовжує зростати, частота гіпертензії та пов’язаних із нею серцево-ниркових захворювань також буде зростати, якщо не будуть розроблені більш ефективні стратегії запобігання ожирінню та його лікування.
Епідеміологія АГ, асоційованої з ожирінням та кардіометаболічним ризиком
Згідно з нещодавно опублікованими оцінками, майже половина (45 %) дорослих у Сполучених Штатах мають АГ, що визначається як систолічний артеріальний тиск (САТ) ≥ 130 мм рт.ст., діастолічний артеріальний тиск (ДАТ) ≥ 80 мм рт.ст., або приймають антигіпертензивні препарати [1, 2]. АГ є основною причиною підвищення смертності, хронічних захворювань нирок та серцево-судинних захворювань (ССЗ), включаючи інфаркт міокарда, серцеву недостатність та інсульт. Підвищена маса тіла та ожиріння є основними факторами ризику гіпертензії та часто виникають при цьому; таким чином, стратегії навмисного зниження ваги є ідеальними цілями для зниження ризику хронічних захворювань і смертності в осіб із надмірною масою тіла/ожирінням та АГ. Виражене вісцеральне ожиріння, а не підшкірне ожиріння більш тісно пов’язане із розвитком АГ [3]. Очікується, що поширеність АГ в популяції буде зростати із зростанням поширеності ожиріння в усьому світі. За оцінками Всесвітньої організації охорони здоров’я, у 2016 році > 1,0 мільярда дорослих мали надлишкову масу тіла і > 650 мільйонів із цих осіб мали ожиріння [4]. Поширеність тяжкого ожиріння серед дорослих у США становила 9,2 % із 2017 по 2018 рік, що на 38 % більше, ніж за 10 років раніше [5]. Крім того, поширеність ожиріння та надмірної маси тіла різко зростає серед дітей та підлітків і вражає > 18 % осіб у всьому світі [4]. Таким чином, АГ, пов’язана із надмірною масою тіла, є зростаючою проблемою, яка помітно вплине на систему охорони здоров’я в усьому світі.
Вплив гіпертензії, асоційованої з ожирінням: ураження органів-мішеней
Ожиріння та АГ тісно пов’язані з ураженням органів-мішеней: судин, серця, нирок та мозку. Дані широкомасштабних когортних досліджень демонструють чіткий дозозалежний взаємозв’язок між більшим ожирінням та більш високим ризиком серцевої недостатності, ішемічної хвороби серця та інсульту [9, 10]. Крім того, у метааналізі пацієнтів — учасників 39 когортних досліджень Chang і співавтори [11] виявили, що індекс маси тіла (ІМТ) 40 кг/м2 порівняно із 25 кг/м2 був пов’язаний із 2-кратним підвищенням ризику зниження функції нирок на 40 % або розвитку термінальної стадії захворювання нирок. Значна втрата маси тіла внаслідок метаболічної хірургії порівняно із звичайним лікуванням приводила до зменшення гіперфільтрації нирок, протеїнурії та ризику серйозних ускладнень ССЗ та термінальної стадії захворювання нирок [12, 13].
Зв’язок між ожирінням і ризиком ураження органів-мішеней зникає після коригування щодо наявності АГ, що свідчить про те, що гіпертензія є ключовим фактором, який пояснює ураження органів-мішеней при ожирінні [10]. У ретроспективному аналізі дослідження SPRINT початково вищий ІМТ не був пов’язаний із більшим впливом інтенсивного контролю АТ на ризик ССЗ [14]. Однак відповідне лікування АГ залишається вирішальним для зменшення негативного впливу ожиріння на органи-мішені.
Патофізіологія гіпертензії, асоційованої з ожирінням
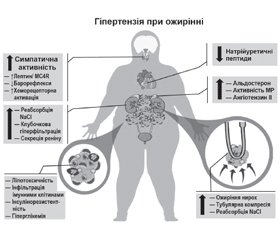
Патофізіологія АГ при ожирінні є багатофакторною й сильно залежить від часу (рис. 1). Перегодовування людей і піддослідних тварин швидко активує симпатичну нервову систему (СНС) і ренін-ангіотензин-альдостеронову систему (РААС), навіть до того, як відбувається значне збільшення маси тіла [15–18]. І навпаки, зниження споживання калорій внаслідок добровільного обмеження їжі або метаболічної хірургії швидко знижує артеріальний тиск (АТ) і послаблює метаболічні розлади в більшості пацієнтів з ожирінням, включаючи хворих на цукровий діабет 2-го типу [15, 19]. Хоча підвищення АТ, яке супроводжує збільшення ваги, спочатку є легким, при хронічному ожирінні відбувається поступове пошкодження органів-мішеней, що загострює гіпертензію. Довгостроковий вплив ожиріння на АТ також залежить від того, де зберігається надлишок жиру, при цьому вісцеральний жир несе більший ризик розвитку АГ, ніж підшкірний жир [20, 21].
Механізми, які викликають АГ при ожирінні
Ожиріння призводить до збільшення об’єму позаклітинної рідини та збільшення кровотоку в багатьох тканинах, що спричинює збільшення венозного повернення та серцевого викиду [16, 22]. Збільшення об’єму опосередковується підвищенням реабсорбції натрію в ниркових канальцях, оскільки спочатку під час розвитку ожиріння нирковий кровотік і швидкість клубочкової фільтрації підвищуються та прогресують до ураження нирок. Принаймні 3 основні фактори сприяють підвищенню реабсорбції натрію: 1) активація РААС, включаючи стимуляцію мінералокортикоїдних рецепторів; 2) активація СНС, особливо підвищення активності ниркового симпатичного нерва; 3) компресія нирки вісцеральним, позачеревним і нирковим синусовим жиром. Декілька інших факторів розглядалися як потенційні медіатори АГ при ожирінні, включаючи резистентність до інсуліну, запалення, дефіцит натрійуретичного гормону, зміни мікробіоти кишечника та збільшення периваскулярної жирової тканини [23, 24]. Однак важливість цих механізмів в ініціації АГ при ожирінні досі не з’ясована.
Активація СНС
Ожиріння викликає активацію СНС, що диференційовано контролюється в різних тканинах і пов’язана в основному зі збільшенням вісцерального ожиріння [25–27]. Підвищення активності СНС зазвичай є помірним і не зменшує тканинний кровотік, але його достатньо для збільшення ниркової реабсорбції натрію та вивільнення реніну. Ниркова денервація помітно послаблює АГ при експериментальному ожирінні, а також у резистентних до лікування пацієнтів з ожирінням [15, 25]. Активації симпатичної системи при ожирінні сприяють численні фактори, включаючи барорефлекторну дисфункцію, гіпоксію та активацію хеморецепторів, особливо в пацієнтів з апное сну. Лептин, адипокін, що секретуються пропорційно до ступеня ожиріння, також стимулють активність СНС при АГ, асоційованій з ожирінням, головним чином активуючи нейрони проопіомеланокортину, які, у свою чергу, активують рецептори меланокортину-4 мозку [15, 28].
Активація РААС
Кілька механізмів, пов’язаних із вісцеральним ожирінням, активують РААС, включаючи компресію нирок та посилення активації СНС [15, 20]. Експериментальні та клінічні дослідження показують, що блокатори РААС ефективні для зниження АТ у пацієнтів з ожирінням, хоча ангіотензин II лише незначно підвищений, що свідчить про підвищену чутливість до ангіотензину II. Антагонізм мінералокортикоїдних рецепторів також знижує АТ і зменшує ураження органів-мішеней при АГ, асоційованій з ожирінням, навіть якщо рівень альдостерону в плазмі є нормальним або субнормальним, що вказує на те, що активація мінералокортикоїдних рецепторів принаймні частково не залежить від альдостерону [29, 30].
Компресія нирок
Із накопиченням вісцерального жиру та жиру в периренальному та нирковому синусах, а також підвищенням внутрішньочеревного тиску нирки стискаються, що ще більше активує РААС, збільшуючи реабсорбцію натрію та сприяючи підвищенню АТ, ініційованому активацією СНС [15, 31].
Запалення, метаболічні розлади та прогресуюче ураження серця і нирок посилюють АГ при ожирінні
Вісцеральне ожиріння ініціює запальні реакції в жировій тканині та органах по всьому організму, включаючи нирки, у результаті активації резидентних макрофагів, інфільтрації макрофагів та секреції прозапальних цитокінів, які діють локально та паракринно/ендокринно [32, 33]. Ектопічне накопичення ліпідів у печінці, скелетних м’язах, нирках, кровоносних судинах та інших органах призводить до ліпотоксичності, запалення та каскаду метаболічних порушень, включаючи дисліпідемію, резистентність до інсуліну, непереносимість глюкози та діабет 2-го типу з часом [15, 32, 33]. Ці хронічні запальні та метаболічні розлади взаємодіють із підвищенням АТ, викликаючи окиснювальний стрес, стрес ендоплазматичного ретикулуму та мітохондріальну дисфункцію в кровоносних судинах, серці та нирках.
На початку ожиріння спостерігаються легкий або помірний фіброз нирок, мікроальбумінурія, розширення мезангіального матриксу, гломеруломегалія, фокальний сегментарний клубочковий склероз і пошкодження подоцитів, пов’язане із підвищеною швидкістю клубочкової метафільтрації (гіперфільтрація клубочків, ожиріння) [34, 35]. Коли запальні розлади зберігаються протягом багатьох років, клубочкова гіперфільтрація зменшується і змінюється зниженням швидкості клубочкової фільтрації та підвищенням чутливості АТ до солі, що пов’язано із втратою нефронів. Ожиріння також посилює шкідливі ефекти інших первинних ушкоджень нирок, таких як одностороння нефректомія, трансплантація нирки та імуноглобулін-А-нефропатія [31]. Із зниженням функції нирок АГ стає більш вираженою, адекват-ний контроль АТ стає більш складним, а ушкодження кровоносних судин в усьому організмі та серця прогресує.
Вплив дієти на стійку втрату маси тіла та контроль АГ
Кілька національних рекомендацій радили здорову дієту окремо або як частину цілісного підходу до здорового способу життя для контролю АГ, контролю маси тіла та зниження ризику ССЗ [2, 6, 36, 37]. Існуючі дієтичні рекомендації акцентують увагу на дієті, а не окремих продуктах та поживних речовинах для профілактики та контролю ССЗ [38]. Найвідомішими моделями здорового харчування є середземноморська дієта та DASH-дієта. Ці 2 схеми харчування так само багаті фруктами, овочами, бобовими, горіхами та насінням, із помірним споживанням риби, морепродуктів, птиці та молочних продуктів і низьким споживанням червоного та обробленого м’яса та солодощів. Середземноморська дієта також сприяє широкому вживанню оливкової олії та регулярному, але помірному споживанню вина (особливо червоного).
Середземноморська дієта
Кокрейнівський огляд рандомізованих контро-льованих досліджень (РКД) 2019 року показав, що середземноморська дієта мала значний сприятливий вплив на САТ (–3,0 мм рт.ст. [95% ДІ від –3,5 до –2,5]) і ДАТ (–2,0 мм рт.ст. [95% ДІ від –2,3 до –1,7]) [39]. Метааналіз 16 РКД показав, що застосування середземноморської дієти також знижує масу тіла (–1,8 кг [95% ДІ від –2,9 до –0,6]) та ІМТ (–0,6 кг/м2 [95% ДІ від –0,9 до –0,2]) [40].
Дієта DASH
Порівняно із середземноморською дієтою дієта DASH, здається, забезпечує більш надійний ефект зниження АТ. У метааналізі 24 РКД дієтичних втручань дієта DASH мала сильний ефект на зниження САТ (–7,6 мм рт.ст. [95% ДІ від –10,0 до –5,3]) і ДАТ (–4,2 мм рт.ст. [95% ДІ від −5,9 до −2,6]) [41]. У поєднанні зі схудненням і фізичними вправами дієта DASH призвела до значно більшого зниження АТ (–16,1/9,9 мм рт.ст.), ніж лише DASH-дієта (–11,2/7,5 мм рт.ст.) [42]. Ефект дієти DASH на зниження АТ також виявився сильнішим у поєднанні із меншим споживанням натрію, особливо в осіб, які страждали на АГ. У дослідженні DASH-Sodium порівняно із дієтою DASH із високим споживанням натрію (3450 мг/день) дієта DASH із низьким споживанням натрію (1150 мг/день) знижувала САТ на 0,9 мм рт.ст. (95% ДІ від –2,1 до –0,3), 3,3 мм рт.ст. (95% ДІ від –4,7 до –1,9), 4,9 мм рт.ст. (95% ДІ від –7,3 до –2,6) і 10,4 мм рт.ст. (95% ДІ від –15,5 до –5,3) у дорослих із початковими рівнями САТ < 130, 130–139, 140–149 і ≥ 150 мм рт.ст. відповідно [43].
Незалежно від режиму харчування, низьке споживання натрію також допомагає контролювати АТ. У метарегресійному аналізі 133 РКД зниження споживання натрію на 2300 мг/добу було пов’язане зі зниженням САТ на 7,7 мм рт.ст. (95% ДІ від –10,4 до –5,0) і 3,0 мм рт.ст. (95% ДІ від –4,6 до –1,4) в осіб із АТ > 131/78 мм рт.ст. [44]. Крім того, результати метааналізу показали, що кожне зниження на 50 ммоль 24-годинного натрію в сечі (об’єктивний показник споживання натрію) було пов’язане із зниженням САТ на 1,10 мм рт.ст. (95% ДІ 0,7–1,5) [45]. Ефекти зниження рівня натрію на АТ, мабуть, особливо очевидні в людей старшого віку, гіпертоніків і темношкірих [45–47]. Підвищене споживання калію також пов’язане зі зниженням АТ, хоча надмірне споживання калію може асоціюватися із несприятливими наслідками [48, 49].
Інтервальне голодування
Окрім цих широко рекомендованих підходів до покращення складу та якості дієти, деякі дослідження вивчали час прийому їжі для схуднення та контролю АТ. Дані невеликих клінічних досліджень у пацієнтів із метаболічним синдромом свідчать про те, що інтервальне голодування може призвести до помірного зниження САТ і ДАТ [50], подібно до зниження, досягнутого при втраті маси тіла за допомогою інших заходів. Систематичний огляд 4 РКД показав, що хоча інтервальне голодування було ефективним для короткочасної втрати маси тіла, його вплив на зниження артеріального тиску був слабким [51]. Інший систематичний огляд та метааналіз 6 РКД показав, що інтервальне голодування було ефективнішим, ніж відсутність лікування, для втрати маси тіла (–4,1 кг [95% ДІ від –2,0 до –6,3]), але істотно не відрізнялося від ефектів постійного обмеження енергії (–1,0 кг [95% ДІ від –2,5 до 0,4]) [52]. Вплив інтервального голодування на контроль АТ потребує подальших досліджень.
Вплив фізичної активності на стійке зниження маси тіла та механізми впливу фізичної активності на контроль АТ
Фізична активність (ФА) визначається як рухи тіла, що виникають внаслідок скорочення скелетних м’язів, що збільшує витрати енергії вище від рівня спокою [53]. Вправи оцінюють за інтенсивністю як помірні, енергійні та інтенсивні, які планують, структурують та повторюють із метою покращення або підтримки здоров’я [53]. Сидячий спосіб життя, що характеризується витратою енергії ≤ 1,5 метаболічного еквіваленту в позі сидячи або лежачи, вважається відмінним від часу ФА помірної або сильної інтенсивності, оскільки обидва стани незалежно пов’язані зі смертністю від усіх причин [54]. Нижче зазначені механізми, які можуть лежати в основі переваг вищих рівнів ФА для контролю АТ та кардіометаболічного ризику.
Імовірні механізми зниження АТ, пов’язані з вищими рівнями фізичної активності
— Втрата маси тіла і зниження ожиріння.
— Підвищення чутливості до інсуліну та сприйняття глюкози.
— Зниження симпатичної активності і підвищення парасимпатичної активності.
— Підвищення барорефлекторної чутливості.
— Зниження судинного опору.
— Підвищення податливості судин.
— Поліпшення функції ендотелію, опосередкованої оксидом азоту.
— Поліпшення жорсткості артерій.
— Зниження окисного стресу.
— Зменшення запалення.
— Зниження рівня ендотеліну-1.
— Менша затримка рідини, що знижує ризик обструктивного апное сну.
Вплив ФА на гіпертензію при ожирінні
Існують докази того, що ФА та фізичні вправи (ФВ) зменшують ожиріння, АТ та гіпертензію при ожирінні. Хоча зменшення факторів ризику ССЗ відзначається при втраті маси тіла уже на 2–3 %, існуючі рекомендації вказують на необхідність зниження маси тіла принаймні на 5–10 % (клінічно значуща втрата маси тіла) протягом 6 місяців, оскільки в такому випадку відбувається більш значуще зниження факторів ризику ССЗ, включаючи ліпіди, та інших відповідних кардіометаболічних факторів ризику, включаючи чутливість до інсуліну, жорсткість артерій та рівень АТ у стані спокою [55]. ФВ також достовірно впливають на АТ незалежно від маси тіла. Нещодавно Noone та співавтори [56] провели метааналіз 93 РКД і показали, що як ФВ, так і ліки ефективно знижували АТ. Хоча оцінка розвитку кінцевих точок показала перевагу ліків порівняно з ФВ, ця різниця не мала статистичної достовірності. З огляду на низьку вартість та відсутність серйозних побічних ефектів або взаємодії із ліками на додаток до впливу ФВ на підвищення рівня кардіореспіраторної працездатності, можливо, одного з найсильніших маркерів ризику ССЗ, ФВ мають бути частиною всіх заходів боротьби з гіпертензією та зниження маси тіла.
Досягнення втрати маси тіла на 5–10 % може призвести до зниження САТ і ДАТ на > 5 та 4 мм рт.ст. відповідно, а втрата 10 кг маси тіла може знизити САТ на 5–20 мм рт.ст. [55, 57]. Як правило, ФА < 150 хв/тиждень призводить до мінімальної втрати ваги, ФА від 150 до 225 хв/тиждень — від 2 до 3 кг, а ФА від 225 до 420 хв/тиждень призводить до втрати маси тіла від 5 до 7,5 кг, і для довготривалого підтримання зниження маси тіла необхідна ФА 200–300 хв/тиждень [55]. Тренування з опором не сприяють клінічно значущій втраті маси тіла, хоча мають помірний вплив на покращення АТ та сприяють корисним змінам будови тіла [55], а також тренування з опором та м’язова сила впливають на зниження смертності від ССЗ незалежно від ФА/кардіореспіраторної здатності [58].
Зменшення малорухомості для зниження АТ
Існують переконливі докази того, що втручання з ФА знижують АТ. Систематичний огляд 26 досліджень показав, що застосування крокомірів підвищувало ФА та знижувало САТ та ДАТ у дорослих амбулаторних пацієнтів [59]. Збільшення кількості доказів, отриманих в інтервенційних дослідженнях, свідчить про те, що зменшення сидячого режиму (тобто скорочення або переривання часу сидіння ходьбою або стоянням) призводить до зниження САТ або ДАТ у діапазоні від 1 до 16 мм рт.ст. [60–65]. Величина зниження АТ із перервами в сидячому положенні може бути більшою у хворих на АГ порівняно з особами без гіпертензії. Через складний взаємозв’язок між ФА та сидячим способом життя настанови зараз не дають конкретних рекомендацій щодо того, на скільки потрібно скоротити час сидіння, щоб спостерігати зниження АТ [66].
Довгострокова ефективність дієти та ФА для контролю АТ
Хоча модифікація дієти, фізичні вправи та пов’язана з цим втрата маси тіла є ефективними стратегіями зниження АТ, імовірність рецидиву АГ залишається високою серед тих, хто дотримується таких змін способу життя. Згідно з оглядом проспективних досліджень, сприятливий вплив втрати маси тіла на АТ значно зменшується або змінюється [67]. Більша частина випадків такого рецидиву пов’язана із звичайним явищем відновлення маси тіла. Наприклад, дослідження ІІ фази TOHP (Trials of Hypertension Prevention) продемонструвало, що в дорослих із помірно підвищеним АТ втрата маси тіла або зменшення споживання натрію знижували АТ, але цей ефект із часом послаблювався [68]. Ретроспективний аналіз дослідження TOHP-II у групі пацєнтів, які схудли, показав, що ті, хто підтримував втрату маси, мали на 65 % нижчу ймовірність розвитку АГ, ніж учасники, рандомізовані до контрольної групи зі звичайним лікуванням [69]. Кілька складних фізіологічних адаптацій до втрати маси тіла сприяють її відновленню, включаючи підвищення енергоефективності, пов’язане із зниженням швидкості метаболізму в стані спокою і зниженням енергетичних витрат, а також зниження відчуття насичення внаслідок гормональних змін [70]. Тому успішне зниження маси тіла протягом багатьох років зазвичай вимагає високого рівня ФА та обмеження часу сидіння, частого контролю маси та дотримання дієти із високим рівнем обмежень [71].
Повернення підвищеного АТ в осіб, які вживають заходів щодо зміни способу життя, також частково не залежить від відновлення маси тіла. Наприклад, у відповідь на дієту 800 калорій на добу протягом 9 тижнів маса тіла знизилася серед 34 чоловіків і жінок у середньому з 101,7 до 87,3 кг (–14,4 кг), а 24-годинний амбулаторний САТ знизився зі 130,1 до 121,1 мм рт.ст. (–9 мм рт.ст.) [72]. Однак, незважаючи на повне збереження втрати маси через 6 місяців, середній 24-годинний САТ зріс до 126,5 мм рт.ст. (–3,6 мм рт.ст. від початкового рівня, 40 % від початкової відповіді). Аналогічно хоча ≈ 88 % початкової втрати маси було збережено протягом 1 року (–12,6 кг), САТ підвищився до 127,9 мм рт.ст. (–2,2 мм рт.ст. від початкового рівня, ≈ 24 % від початкової відповіді АТ). Деякі фізіологічні зміни, які сприяють зниженню АТ в умовах втрати маси тіла, включаючи зниження активності СНС та активності реніну плазми, є тимчасовими навіть у тих, у кого залишається стійка втрата маси тіла [73]. Це свідчить про те, що нестійкі нейрогормональні реакції на втрату маси можуть додатково сприяти рецидиву АГ в осіб, які досягли втрати маси тіла шляхом зміни способу життя.
Фармакотерапія
Препарати для схуднення, схвалені Управлінням з контролю за продуктами і ліками США
Фармакотерапія може бути розглянута для контролю маси тіла в пацієнтів, які мають обмежену терапевтичну відповідь лише на зміни способу життя та мають ІМТ ≥ 30 кг/м2 або ІМТ ≥ 27 кг/м2 за наявності супутніх захворювань, пов’язаних із надмірною масою тіла, таких як АГ [6]. Призначена фармакотерапія проти ожиріння використовується як доповнення до дієти та фізичних вправ. Управлінням з контролю за продуктами і ліками США (FDA) схвалені лише чотири види медикаментозної терапії і тільки для короткочасного використання (до 12 тижнів) для лікування ожиріння: фентермін, діетилпропіон, фендиметразин та бензфетамін. Ці ліки мають тісний структурний і механістичний зв’язок з амфетаміном [74]. Зараз FDA схвалило ще 5 лікарських засобів для тривалої втрати ваги: орлістат, фентермін/топірамат подовженого вивільнення, налтрексон/бупропіон, ліраглутид 3,0 мг і семаглутид 2,4 мг щотижня підшкірно. Основним механізмом дії орлістату є зниження всмоктування жиру в кишечнику [74]. Фентермін/топірамат, налтрексон/бупропіон та ліраглутид є препаратами центральної дії, які підвищують насичення і зменшують відчуття голоду. У РКД препарати, схвалені для тривалого застосування (разом зі зміною способу життя), зменшують масу тіла в середньому на 3–9 % більше, ніж плацебо (зі зміною способу життя), протягом 1 року [75]. Втрата маси за перші 3–4 місяці після початку фармакотерапії є найбільш послідовним предиктором 1-річної відповіді на ці препарати і може використовуватися як керівництво для продовження фармакотерапії або переходу на альтернативну стратегію зниження ваги [76]. Пацієнти зі стійкою втратою маси тіла та ті, які ці препарати добре переносять, можуть отримати користь від тривалого застосування фармакотерапії проти ожиріння. Відновлення маси або додаткове її збільшення іноді спостерігається, якщо ці препарати продовжують приймати більше 1 року або після припинення їх прийому [74].
Довгострокові ефекти ліків від ожиріння на АТ є неоднозначними через різні фактори, включаючи відмінності в механізмах дії, ефективності зниження маси тіла та досліджуваних популяціях. РКД демонструють незначне зниження АТ у пацієнтів, рандомізованих на прийом орлістату, фентерміну/топірамату і ліраглутиду, порівняно із плацебо через 1 рік (середнє зниження САТ на 1–3 мм рт.ст. і зниження ДАТ на 1 мм рт.ст.; табл. 1); вважається, що це опосередковано втратою маси тіла [75, 76]. Крім того, РКД демонструють невелике підвищення АТ у пацієнтів, рандомізованих на прийом налтрексону/бупропіону, порівняно з плацебо (середнє збільшення САТ на 2 мм рт.ст. і збільшення ДАТ на 1 мм рт.ст.) [76]. Слід зазначити, що в цьому РКД лише підгрупа тривалого застосування препаратів проти ожиріння мала АГ на початковому етапі. Для препаратів проти ожиріння, які продемонстрували зниження АТ, таке зниження є дещо більшим (у середньому на 1 мм рт.ст.) у підгрупі учасників з основним діагнозом стабільної АГ [75, 76]. У нещодавньому дослідженні підшкірна ін’єкція семаглутиду 2,4 мг 1 раз на тиждень протягом 68 тижнів як доповнення до змін способу життя призвела до зниження середньої маси тіла на 14,9 % і зниження САТ на 6,2 мм рт.ст. від вихідного рівня. Цей препарат, спочатку розроблений для лікування цукрового діабету, є перспективним для більшого, ніж у середньому, зниження маси тіла із потенційно більшим покращенням кардіометаболічного ризику, особливо при застосуванні препарату в поєднанні з втручаннями, направленими на зміни способу життя [77].
/22.jpg)
Безпека та ускладнення
Пацієнтів слід попередити про потенційні побічні ефекти фармакотерапії проти ожиріння перед застосуванням. Найпоширенішими побічними ефектами від симпатоміметичних амінів, таких як фентермін, є запор, запаморочення, сухість у роті та безсоння [74]. Існує також потенційний ризик підвищення АТ, пов’язаний із механізмом дії, який включає підвищення рівня катехоламінів. Хоча етикетки FDA попереджають про цей ризик для препаратів, схвалених для тривалої втрати ваги (наприклад, комбінація фентермін/топірамат), у РКД цих препаратів клінічно значущого підвищення АТ не спостерігалося [76], що може бути пов’язане з супутньою втратою маси тіла. Орлістат має загалом сприятливий профіль безпеки, що пояснюється його периферичним механізмом дії. Однак він часто погано переноситься через високу частоту рідких випорожнень, імперативних позивів до випорожнення та метеоризму, коли пацієнти суворо не дотримуються дієти з низьким вмістом жиру. Пацієнти, яким призначений орлістат, повинні приймати полівітаміни через зниження всмоктування жиророзчинних вітамінів. Лоркасерин був раніше схвалений FDA для тривалого застосування для схуднення, але був добровільно вилучений з ринку США в лютому 2020 року через підвищену захворюваність на рак (включаючи рак підшлункової залози, колоректальний рак та рак легень), яка спостерігалася серед пацієнтів, які приймали лоркасерин, порівняно із плацебо під час 5-річнного спостереження в РКД [78]. При аналізі національної вибірки даних електронних медичних карток Zhang та співавтори [79] помітили, що < 1 % із майже 2 мільйонів відповідних пацієнтів отримували фармакотерапію для зниження ваги. Низька частота призначення може бути пов’язана із більшим ризиком, ніж користю, для багатьох клініцистів, враховуючи мінімальний вплив на АТ та відсутність даних про довгостроковий вплив цих препаратів на ураження органів-мішеней.
Метаболічна хірургія
Баріатричні операції
Загалом у Сполучених Штатах у 2016 році було проведено 216 000 процедур метаболічної хірургії (МХ) [80]. Рукавна резекція шлунка є найпоширенішою метаболічною процедурою (58 %), за нею йдуть шунтування шлунка за типом Roux-en-Y (RYGB; 19 %), регульований бандаж шлунка (3 %) і біліопанкреатичне відведення із дуоденальним перемикачем (0,6 %). В даний час > 98 % процедур МХ виконується лапароскопічно [81] із частотою великих періопераційних ускладнень < 5 %, смертністю < 0,2 %, перебуванням у стаціонарі від 1 до 2 днів і відновленням через 2–4 тижні. Загалом ці операції включають певний ступінь зменшення об’єму шлунка або кишкове шунтування. Вважається, що втрата ваги та метаболічні поліпшення насамперед обумовлені нейроендокринними механізмами, які знижують апетит і підсилюють відчуття насичення, а також покращують чутливість до інсуліну та його секрецію. Крім того, процедури кишкового шунтування призводять до зниження всмоктування калорій у кишках, що ще більше зменшує загальне споживання калорій.
Поточні показання
Пацієнти із ІМТ ≥ 40 кг/м2 або ≥ 35 кг/м2 із супутніми захворюваннями є кандидатами на МХ, якщо вони психологічно стабільні та не зловживають активними речовинами [82]. Пацієнти із цукровим діабетом 2-го типу та ІМТ ≥ 30 кг/м2 (≥ 27,5 кг/м2 для пацієнтів, які є азіатами) є кандидатами на МХ, якщо вони не мають належного глікемічного контролю на фоні раціональної медикаментозної терапії [83]. МХ слід проводити в центрах із багатопрофільною командою, яка включає баріатричного хірурга, ендокринолога/діабетолога, кардіолога, анестезіолога, психолога та дієтолога із досвідом догляду в галузі ожиріння та діабету.
Механізми, за допомогою яких метаболічна хірургія знижує АТ
Механізми покращення контролю АТ після МХ виявляються багатофакторними, складними та недостатньо зрозумілими на даний момент. Зниження АТ відбувається вже через 1 тиждень після операції, тобто до будь-якої значної втрати маси тіла, що свідчить про роль нейроендокринних механізмів. Підвищення рівнів інкретину (а саме глюкагоноподібного пептиду 1) спостерігалося після різних типів МХ. Глюкагоноподібний пептид 1 стимулює постпрандіальну секрецію інсуліну, інгібує секрецію глюкагону та має кілька центральних дій, включаючи гіпофагію [84]. СНС може бути залучена до ефектів зниження АТ при МХ, враховуючи, що area postrema, один із органів, розташованих на дні четвертого шлуночка, містить катехоламінергічні ней-рони, які реагують на глюкагоноподібний пептид 1. Глюкагоноподібний пептид 1 може бути важливим для гомеостазу води та солі, причому його високі рівні пов’язані з натрійурезом. Натрійуретичні пептиди також можуть відігравати певну роль у покращенні контролю АТ, індукованого МХ. Їх концентрації у крові є низькими в пацієнтів з ожирінням і збільшуються після МХ [85]. На гіпертензію можуть також впливати зміни в поліпептидах, що секретуються білою жировою тканиною, тобто цитокінах (адипокінах), а також системне та ниркове запалення [86]. Нарешті, пацієнти із центральним ожирінням мають підвищену активацію РААС, яка може нормалізуватися після операції.
Огляд клінічних досліджень з МХ як втручанням
Систематичні огляди даних спостережень припускають, що МХ може покращувати перебіг АГ. Один приклад (136 досліджень, 22 094 пацієнти) виявив загальне зниження частоти АГ на 63 % із специфічними для процедури відсотками — 68, 43 і 83 % для RYGB, регульованого бандажа шлунка та біліопанкреатичного відведення з дуоденальним перемикачем відповідно [87]. Під час середнього 10-річного спостереження дослідники з дослідження SOS (Swedish Obese Subject) спостерігали достовірно більше зниження як САТ, так і ДАТ у пацієнтів, які перенесли RYGB, порівняно із конт-рольними пацієнтами, які не отримували хірургічного лікування (пацієнти, які отримували RYGB, −5,1 і −5,6 мм рт.ст.; контрольні суб’єкти 1,2 і – 3,8 мм рт.ст.; р < 0,01 для різниці) [88]. Крім того, відсоток пацієнтів, які приймали антигіпертензивні препарати, був значно нижчим у групі RYGB порівняно із контрольною групою (35 проти 53 %; р < 0,001). Однак зниження АТ, яке корелювало зі зниженням ІМТ через 2 роки, не спостерігалося через 10 років. Adams та співавтори [89] виявили, що АТ стабілізувався через 12 років після RYGB, тоді як значне підвищення АТ із часом (стандартизований показник в середньому на > 6 мм рт.ст. для САТ та ДАТ) спостерігалося у 2 контрольних групах без операції (р < 0,05 для всіх порівнянь між групою RYGB та контролем). З іншого боку, РКД, що порівнювали МХ із медикаментозним лікуванням діабету, не виявили значних довгострокових переваг МХ щодо контролю АТ порівняно із медикаментозним лікуванням. Однак Schauer та співавтори [90] відзначили зменшення використання антигіпертензивних засобів після МХ порівняно із медикаментозним лікуванням.
Дослідження GATEWAY
РКД GATEWAY (Gastric Bypass to Treat Obese Patients With Steady Hypertension) є єдиним контро-льованим дослідженням, у якому оцінювали вплив МХ на рівень АТ як первинну кінцеву точку [91]. У дослідженні GATEWAY 100 пацієнтів з ІМТ від 30 до 39,9 кг/м2, які отримували лікування ≥ 2 антигіпертензивними засобами в максимальних дозах або > 2 засобами в помірних дозах, були рандомізовані 1 : 1 або для RYGB плюс медикаментозна терапія (n = 50) або тільки для медикаментозної терапії (n = 50). Первинний результат: через 12 місяців спостерігалося зниження загальної кількості антигіпертензивних препаратів на ≥ 30 %, необхідних для підтримки офісного АТ < 140/90 мм рт.ст., був більш поширеним у групі RYGB порівняно із контрольною групою (83,7 проти 12,8 %; коефіцієнт ризику 6,6 [95% ДІ 3,1–14,0]; р < 0,001). Ремісія АГ, визначена як рівень АТ < 140/90 мм рт.ст. без застосування ліків, була більш поширеною після RYGB (51 проти 0 %). За 3 роки спостереження в дослідженні GATEWAY первинний результат спостерігався у 73 % пацієнтів із групи RYGB порівняно з 11 % пацієнтів в групі медикаментозної терапії (відносний ризик 6,52 [95% ДІ 2,50–17,03]; р < 0,001) [92]. Ремісія АГ (35 проти 2 %) та застосування ліків (медіана 1 [міжквартильний діапазон 0–2] проти 3 [міжквартильний діапазон 2,8–4]) указували на переваги застосування RYGB порівняно з медикаментозною терапією (р < 0,001). Загальна втрата маси тіла становила 27,8 та 0,1 % у групах RYGB та медикаментозної терапії відповідно. У групі RYGB у 13 пацієнтів розвинувся гіповітаміноз В12, а 2 пацієнтам була потрібна повторна операція.
Безпека та ускладнення
Відтоді, як малоінвазивна хірургія була запроваджена в 1990-х роках, відбулося значне зниження періопераційної захворюваності та смертності, пов’язаної з МХ. Загальнонаціональна база даних стаціонарних хворих США показала загальний рівень внутрішньолікарняної захворюваності 9 % і ризик смертності 0,1 % [93]. Систематичний огляд літератури показав, що частота періопераційних ускладнень серед пацієнтів, які перенесли МХ, коливалася від 10 до 17 %, а 30-добовий рівень смертності становив 0,08 % [94]. Частота періопераційних ускладнень МХ приблизно еквівалентна такому ж показнику при лапароскопічній холецистектомії або лапароскопічній апендектомії або гістеректомії [95].
Профілактика гіпертензії ожиріння
Профілактика збільшення маси тіла та ожиріння має першорядне значення для запобігання кардіометаболічним захворюванням, включаючи АГ та подільші захворювання серця, нирок і мозку. Поширеність ожиріння в дітей та підлітків у віці від 2 до 19 років становила 18,5 %, воно вражало ≈ 13,7 мільйонів осіб з 2015 по 2016 рік [96]. У дітей та підлітків збільшення ІМТ сильно корелює зі збільшенням АТ. Діти з ожирінням мають у 2 рази підвищений ризик розвитку АГ, а діти з тяжким ожирінням мають більше ніж 4-кратний ризик розвитку гіпертензії порівняно з дітьми, які підтримують нормальну вагу. Тому необхідні комплексні та скоординовані зусилля, включаючи мультидисциплінарні стратегії в системах охорони здоров’я, залучення пропаганди та асоціації споживачів, дослідницькі, освітні, медіапрограми [97]. Інші фактори, включаючи ожиріння та АГ у матері та батька, гіпертензивні прояви під час вагітності та генетичні причини раннього ожиріння, можуть відігравати важливу роль у розвитку АГ при ожирінні пізніше в житті і є галузями, які потребують подальшого дослідження [98, 99].
Запитання, на які немає відповіді, та напрямки на майбутнє
На рис. 2 і в тексті нижче висвітлені прогалини в дослідженні та запитання, на які немає відповідей, із пропозиціями щодо майбутніх напрямків.
Питання, на які немає відповіді, та майбутні напрямки
— Які нові стратегії та науково обґрунтовані рекомендації необхідні для запобігання дитячому ожирінню та АГ?
— Чи запобігає навмисна втрата маси тіла за допомогою фармакотерапії або МХ у дитинстві та ранньому дорослому віці АГ та подальшому ураженню органів-мішеней у подальшому житті?
— Яку оптимальну кількість часу мають виділити клініцисти, перш ніж рекомендувати більш агресивні стратегії контролю ваги (наприклад, ліки від ожиріння або МХ), крім зміни способу життя?
— Яку оптимальну кількість часу мають виділити клініцисти, перш ніж рекомендувати більш агресивні стратегії лікування АГ, крім зміни способу життя?
— Чи є фармакотерапія або МХ проти ожиріння корисними для пацієнтів із поширеними ССЗ, включаючи СН (особливо СН зі збереженою фракцією викиду)?
— У який момент або в якому віці лікування схудненням, крім дієти та фізичних вправ, потенційно перевищує точку користі? Чи є шкода, пов’язана із фармакотерапією проти ожиріння або МХ, у літніх людей?
— Які є можливості для виявлення та стратифікації пацієнтів з ожирінням, у яких може бути підвищений ризик ураження органів-мішеней і ризик кардіометаболічних або ниркових захворювань у подальшому?
— Чи слід розглядати фармакотерапію проти ожиріння або МХ для менш тяжкого ожиріння (тобто при ІМТ < 35 кг/м2) або для тих, хто має надлишкову вагу із раннім ураженням органів-мішеней (наприклад, гіпертрофією лівого шлуночка або ХХН)?
— Потрібні подальші дослідження, щоб вивчити, наскільки і в який спосіб негативні соціальні детермінанти здоров’я та нерівність у доступі до медичної допомоги негативно впливають на пов’язані з ожирінням показники здоров’я серед недостатньо представлених расових та етнічних груп.
— Чи допомагають заходи, спрямовані на значне зниження рівня анорексигенних гормонів і зниження енергетичних витрат, які супроводжують втрату маси тіла, підтримувати сприятливий вплив втрати маси на АТ?
— Необхідні РКД, що оцінюють МХ та фармакотерапію для зниження ризику ХХН, СН та інсульту.
— Незрозуміло, чи відрізняються механізми, що пояснюють сприятливий вплив зменшення малорухомого способу життя на АТ, від механізмів, що лежать в основі зниження АТ із збільшенням ФА.
— Які мінімальні та максимальні переносимі терміни щодо скорочення часу сидіння для зниження АТ?
— Які механізми материнського та батьківського програмування раннього ожиріння та АГ у потомства?
— Чи ефективні заходи контролю маси тіла під час критичних періодів вагітності в майбутніх матерів і в постнатальному житті їх нащадків у запобіганні дитячому ожирінню та супутнім кардіоренальним та метаболічним розладам?
— Які довгострокові наслідки МХ у підлітків з ожирінням для запобігання кардіоренальним та метаболічним захворюванням у подальшому житті? Наскільки переваги МХ у цих підлітків переважають ризики?
— Чому МХ викликає швидке зниження АТ та порушення обміну речовин ще до значного зниження маси тіла або ожиріння?
Висновки
Ожиріння є основною причиною АГ та подальших серцево-судинних, ниркових і мозкових уражень. Механізми зниження АТ після схуднення можуть бути викликані здебільшого зміною механізмів, що опосередковують підвищення АТ із збільшенням ваги. Однак деякі із цих механізмів, як, наприклад, зниження симпатичної активності, здається, швидко вичерпуються зі зменшенням споживання калорій, навіть при значній втраті маси тіла. Стратегії навмисного схуднення, включаючи зміни способу життя, такі як дієта, збільшення ФА та зменшення малорухливого способу життя, є важливими методами зниження АТ в осіб з ожирінням, що може зменшити ризики АГ та пов’язаних із нею захворювань. Однак ці зміни способу життя багатьом пацієнтам складно підтримувати, а швидкість відновлення маси тіла висока. Доказові методи лікування, такі як фармакотерапія та МХ, можливо використовувати для лікування ожиріння і, як наслідок, зниження АТ. Ліки проти ожиріння доступні для короткого та тривалого використання; однак рівень виписування рецептів на ці препарати залишається низьким, ймовірно, через обмежене страхове покриття та низький рівень кваліфікації лікарів у лікуванні ожиріння. Коли фармакотерапія проти ожиріння призначається особам із ризиком або з АГ, важливо враховувати механізм дії при визначенні варіанта лікування. МХ є ефективним довгостроковим способом зниження ожиріння в осіб із тяжкими його формами. Крім того, МХ має як короткостроковий, так і важливий довгостроковий вплив на зниження АТ у пацієнтів з ожирінням. Необхідні додаткові РКД для оцінки впливу МХ на зниження ризику розвитку захворювань, пов’язаних з ожирінням, таких як хронічна хвороба нирок, інсульт та серцева недостатність.
Інформація про статтю
Американська кардіологічна асоціація докладає всіх зусиль, щоб уникнути будь-яких фактичних або потенційних конфліктів інтересів, які можуть виникнути в результаті зовнішніх стосунків або особистих, професійних або ділових інтересів члена комісії. Зокрема, усі члени групи, які пишуть, повинні заповнити та подати анкету про розкриття інформації, яка показує всі такі стосунки, які можуть бути сприйняті як реальний або потенційний конфлікт інтересів.
Цю заяву схвалили Науково-консультативний та координаційний комітет Американської асоціації серця 7 червня 2021 року та Виконавчий комітет Американської асоціації серця 21 червня 2021 року. Копія документа доступна за адресою https://professional.heart.org/записи за допомогою «Пошук рекомендацій та заяв» або область «Огляд за темою».
Переклад Ю. Сіренка
Оригінал статті надрукований
в журналі Hypertension. 2021. 78. e38-e50.
Список литературы
1. Centers for Disease Control and Prevention (CDC). Hypertension Cascade: Hypertension Prevalence, Treatment and Control Estimates Among US Adults Aged 18 Years and Older Applying the Criteria From the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline — NHANES 2013–2016. US Department of Health and Human Services, 2019.
2. Whelton P.K., Carey R.М., Aronow W.S. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the Ame-rican College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Hypertension. 2018. 71. e140-e141]. Hypertension. 2018. 71. e13-e115. doi: 10.1161/HYP.0000000000000065
3. Chandra A., Neeland I.J., Berry J.D. et al. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J. Am. Coll. Cardiol. 2014. 64. 997-1002. doi: 10.1016/j.jacc.2014.05.057
4. World Health Organization. Fact sheet: obesity and overweight. Accessed July 31, 2020. https://www.who.int/news-room/fact-sheets/detail/obesityand-overweight
5. Hales C.М., Carroll M.D., Fryar C.D., Ogden C.L. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics. 2020. Accessed September 13, 2021. https://www.cdc.gov/nchs/products/databriefs/db360.htm
6. Jensen M.D. et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society [published correction appears in Circulation. 2014. 129(suppl. 2). S139-S140]. Circulation. 2014. 129(suppl. 2). S102-S138. doi: 10.1161/01.cir.0000437739.71477.ee
7. Apovian C.М. et al.; on behalf of the Endocrine Society. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015. 100. 342-362. doi: 10.1210/jc.2014-3415
8. Bray G.A. et al. The science of obesity management: an Endocrine Society scientific statement. Endocr. Rev. 2018. 39. 79-132. doi: 10.1210/er.2017-00253
9. Aune D., Sen A., Norat T., Janszky I., Romundstad P., Tonstad S., Vatten L.J. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of рrospective studies. Circulation. 2016. 133. 639-649. doi: 10.1161/CIRCULATIONAHA.115.016801
10. Lu Y., Hajifathalian K., Ezzati M., Woodward M., Rimm E.В., Danaei G.; Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014. 383. 970-983. doi: 10.1016/S0140-6736(13)61836-X
11. Chang A.R. et al.; CKD Prognosis Consortium (CKD-PC). Adiposity and risk of decline in glomerular filtration rate: meta-analysis of individual participant data in a global consortium. BMJ. 2019. 364. k5301. doi: 10.1136/bmj.k5301
12. Li K., Zou J., Ye Z., Di J., Han X., Zhang H., Liu W., Ren Q., Zhang P. Effects of bariatric surgery on renal function in obese patients: a systematic review and meta analysis. PLoS One. 2016. 11. e0163907. doi: 10.1371/journal.pone.0163907
13. Sjöström L. et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012. 307. 56-65. doi: 10.1001/jama.2011.1914
14. Oxlund C. et al. Body mass index, intensive blood pressure management, and cardiovascular events in the SPRINT trial. Am. J. Med. 2019. 132. 840-846. doi: 10.1016/j.amjmed.2019.01.024
15. Hall J.Е., do Carmo J.М., da Silva A.А., Wang Z., Hall M.E. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat. Rev. Nephrol. 2019. 15. 367-385. doi: 10.1038/s41581-019-0145-4
16. Hall J.Е., Brands M.W., Dixon W.N., Smith M.J. Jr. Obesity-induced hypertension. Renal function and systemic hemodynamics. Hypertension. 1993. 22. 292-299. doi: 10.1161/01.hyp.22.3.292
17. O’Dea K., Esler M., Leonard P., Stockigt J.R., Nestel P. Noradrenaline turnover during under- and over-eating in normal weight subjects. Metabolism. 1982. 31. 896-899. doi: 10.1016/0026-0495(82)90178-0
18. Gentile C.L., Orr J.S., Davy B.М., Davy K.P. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007. 292. R1834-R1838. doi: 10.1152/ajpregu.00876.2006
19. Schiavon C.A. et al. Effects of bariatric surgery versus medical therapy on the 24-hour ambulatory blood pressure and the prevalence of resistant hypertension. Hypertension. 2019. 73. 571-577. doi: 10.1161/HYPERTENSIONAHA.118.12290
20. Hall J.Е., do Carmo J.М., da Silva А.А., Wang Z., Hall M.E. Obesityinduced hypertension: interaction of neurohumoral and renal mechanisms. Circ. Res. 2015. 116. 991-1006. doi: 10.1161/CIRCRESAHA.116.305697
21. Piché M.Е., Tchernof A., Després J.P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020. 126. 1477-1500. doi: 10.1161/CIRCRESAHA.120.316101
22. Messerli F.Н. et al. Obesity and essential hypertension: hemodynamics, intravascular volume, sodium excretion, and plasma renin activity. Arch. Intern. Med. 1981. 141. 81-85. doi: 10.1001/archinte.141.1.81
23. Saxton S.N., Clark B.J., Withers S.B., Eringa E.С., Heagerty A.M. Mechanistic links between obesity, diabetes, and blood pressure: role of perivascular adipose tissue. Physiol. Rev. 2019. 99. 1701-1763. doi: 10.1152/physrev.00034.2018
24. da Silva A.А. et al. Role of hyperinsulinemia and insulin resistance in hypertension: metabolic syndrome revisited. Can. J. Cardiol. 2020. 36. 671-682. doi: 10.1016/j.cjca.2020.02.066
25. Lohmeier T.Е., Hall J.E. Device-based neuromodulation for resistant hypertension therapy. Circ. Res. 2019. 124. 1071-1093. doi: 10.1161/CIRCRESAHA.118.313221
26. Lambert E., Straznicky N., Schlaich M., Esler M., Dawood T., Hotchkin E., Lambert G. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007. 50. 862-868. doi: 10.1161/HYPERTENSIONAHA.107.094649
27. Alvarez G.Е., Beske S.D., Ballard T.Р., Davy K.P. Sympathetic neural activation in visceral obesity. Circulation. 2002. 106. 2533-2536. doi: 10.1161/01.cir.0000041244.79165.25
28. do Carmo J.М. et al. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopio-melanocortin neurons. Hypertension. 2011. 57. 918-926. doi: 10.1161/HYPERTENSIONAHA.110.161349
29. de Paula R.В., da Silva A.А., Hall J.E. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension. 2004. 43. 41-47. doi: 10.1161/01.HYP.0000105624.68174.00
30. Williams B. et al.; British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, doubleblind, crossover trial. Lancet. 2015. 386. 2059-2068. doi: 10.1016/S0140-6736(15)00257-3
31. Hall M.Е. et al. Obesity, hypertension, and chronic kidney disease. Int. J. Nephrol. Renovasc. Dis. 2014. 7. 75-88. doi: 10.2147/IJNRD.S39739
32. Zhu Q., Scherer P.E. Immunologic and endocrine functions of adipose tissue: implications for kidney disease. Nat. Rev. Nephrol. 2018. 14. 105-120. doi: 10.1038/nrneph.2017.157
33. Whaley-Connell A., Sowers J.R. Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int. 2017. 92. 313-323. doi: 10.1016/j.kint.2016.12.034
34. Henegar J.R. et al. Functional and structural changes in the kidney in the early stages of obesity. J. Am. Soc. Nephrol. 2001. 12. 1211-1217. doi: 10.1681/ASN.V1261211
35. Kambham N., Markowitz G.S., Valeri A.М., Lin J., D’Agati V.D. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001. 59. 1498-1509. doi: 10.1046/j.1523-1755.2001.0590041498.x
36. LeFevre M.L.; on behalf of the U.S. Preventive Services Task Force. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014. 161. 587-593. doi: 10.7326/M14-1796
37. Arnett D.K. et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published corrections appear in Circulation. 2019. 140. e649-e650, Circulation. 2020. 141. e60, and Circulation. 2020. 141. e771]. Circulation. 2019. 140. e596-e646. doi: 10.1161/CIR.0000000000000678
38. Van Horn L. et al.; on behalf of the American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Stroke Council. Recommended dietary pattern to achieve adherence to the American Heart Association/American College of Cardiology (AHA/ACC) guidelines: a scientific statement from the American Heart Association [published correction appears in Circulation. 2016. 134. e534]. Circulation. 2016. 134. e505-e529. doi: 10.1161/CIR.0000000000000462
39. Rees K., Takeda A., Martin N. et al. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2019. 3. CD009825. doi: 10.1002/14651858.CD009825.pub3
40. Esposito K., Kastorini C.М., Panagiotakos D.В., Giugliano D. Mediterranean diet and weight loss: meta-analysis of randomized controlled trials. Metab. Syndr. Relat. Disord. 2011. 9. 1-12. doi: 10.1089/met.2010.0031
41. Gay H.С., Rao S.G., Vaccarino V., Ali M.K. Effects of different dietary interventions on blood pressure: systematic review and meta-analysis of randomized controlled trials. Hypertension. 2016. 67. 733-739. doi: 10.1161/HYPERTENSIONAHA.115.06853
42. Blumenthal J.А. et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch. Intern. Med. 2010. 170. 126-135. doi: 10.1001/archinternmed.2009.470
43. Juraschek S.Р., Miller E.R. 3rd, Weaver C.М., Appel L.J. Effects of sodium reduction and the DASH diet in relation to baseline blood pressure. J. Am. Coll. Cardiol. 2017. 70. 2841-2848. doi: 10.1016/j.jacc.2017.10.011
44. Graudal N., Hubeck-Graudal T., Jürgens G., Taylor R.S. Dose-response relation between dietary sodium and blood pressure: a meta-regression analysis of 133 randomized controlled trials. Am. J. Clin. Nutr. 2019. 109. 1273-1278. doi: 10.1093/ajcn/nqy384
45. Huang L. et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020. 368. m315. doi: 10.1136/bmj.m315
46. Sacks F.M. et al.; DASHSodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet: DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001. 344. 3-10. doi: 10.1056/NEJM200101043440101
47. Mozaffarian D. et al.; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Global sodium consumption and death from cardiovascular causes. N. Engl. J. Med. 2014. 371. 624-634. doi: 10.1056/NEJMoa1304127
48. Filippini T. et al. Potassium intake and blood pressure: a dose-response meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2020. 9. e015719. doi: 10.1161/JAHA.119.015719
49. Cook N.R. et al.; Trials of Hypertension Prevention Collaborative Research Group. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch. Intern. Med. 2009. 169. 32-40. doi: 10.1001/archinternmed.2008.523
50. Wilkinson M.J. et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell. Metab. 2020. 31. 92-104.e5. doi: 10.1016/j.cmet.2019.11.004
51. Ganesan K., Habboush Y., Sultan S. Intermittent fasting: the choice for a healthier lifestyle. Cureus. 2018. 10. e2947. doi: 10.7759/cureus.2947
52. Harris L. et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database System. Rev. Implement Rep. 2018. 16. 507-547. doi: 10.11124/JBISRIR-2016-003248
53. Diaz K.М., Shimbo D. Physical activity and the prevention of hypertension. Curr. Hypertens. Rep. 2013. 15. 659-668. doi: 10.1007/s11906-013-0386-8
54. Powell K.E. et al. The scientific foundation for the Physical Activity Guidelines for Americans. 2nd еdition. J. Phys. Act. Health. 2019. 16. 1-11. doi: 10.1123/jpah.2018-0618
55. Swift D.L. et al. The effects of exercise and physical acti-vity on weight loss and maintenance. Prog. Cardiovasc. Dis. 2018. 61. 206-213. doi: 10.1016/j.pcad.2018.07.014
56. Noone C. et al. Comparative efficacy of exercise and antihypertensive pharmacological interventions in reducing blood pressure in people with hypertension: a network meta-analysis. Eur. J. Prev. Cardiol. 2020. 27. 247-255. doi: 10.1177/2047487319879786
57. Ozemek C. et al. Nonpharmacologic management of hypertension: a multidisciplinary approach. Curr. Opin. Cardiol. 2017. 32. 381-388. doi: 10.1097/HCO. 0000000000000406
58. Liu Y. et al. Associations of resistance exercise with cardiovascular disease morbidity and mortality. Med. Sci. Sports Exerc. 2019. 51. 499-508. doi: 10.1249/MSS.0000000000001822
59. Bravata D.М. et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007. 298. 2296-2304. doi: 10.1001/jama.298.19.2296
60. Bhammar D.M., Sawyer B.J., Tucker W.J., Gaesser G.A. Breaks in sitting time: effects on continuously monitored glucose and blood pressure. Med. Sci. Sports Exerc. 2017. 49. 2119-2130. doi: 10.1249/MSS.0000000000001315
61. Champion R.В., Smith L.R., Smith J., Hirlav B., Maylor B.D., White S.L., Bailey D.P. Reducing prolonged sedentary time using a treadmill desk acutely improves cardiometabolic risk markers in male and female adults. J. Sports Sci. 2018. 36. 2484-2491. doi: 10.1080/02640414.2018.1464744
62. Larsen R.N. et al. Breaking up prolonged sitting reduces resting blood pressure in overweight/obese adults. Nutr. Metab. Cardiovasc. Dis. 2014. 24. 976-982. doi: 10.1016/j.numecd.2014.04.011
63. Zeigler Z.S. et al. Effects of standing and light-intensity activity on ambulatory blood pressure. Med. Sci. Sports Exerc. 2016. 48. 175-181. doi: 10.1249/MSS.0000000000000754
64. Zeigler Z.S., Swan P.D., Bhammar D.М., Gaesser G.A. Walking workstation use reduces ambulatory blood pressure in adults with prehypertension. J. Phys. Act. Health. 2015. 12(suppl. 1). S119-S127. doi: 10.1123/jpah.2013-0487
65. Dempsey P.C. et al. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J. Hypertens. 2016. 34. 2376-2382. doi: 10.1097/HJH.0000000000001101
66. Piercy K.L. et al. The Physical Activity Guidelines for Americans. JAMA. 2018. 320. 2020-2028. doi: 10.1001/jama.2018.14854
67. Semlitsch T. et al. Long-term effects of weight-reducing diets in people with hypertension. Cochrane Database Syst. Rev. 2016. 3. CD008274. doi: 10.1002/14651858.CD008274.pub3
68. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure: the Trials of Hypertension Prevention, phase II: the Trials of Hypertension Prevention Collabo-rative Research Group. Arch. Intern. Med. 1997. 157. 657-667.
69. Stevens V.J. et al.; Trials for the Hypertension Prevention Research Group. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann. Intern. Med. 2001. 134. 1-11. doi: 10.7326/0003-4819-134-1-200101020-00007
70. Melby C.L. еt al. Attenuating the biologic drive for weight regain following weight loss: must what goes down always go back up? Nutrients. 2017. 9. E468. doi: 10.3390/nu9050468
71. Wing R.R., Phelan S. Long-term weight loss maintenance. Am. J. Clin. Nutr. 2005. 82(suppl.). 222S-225S. doi: 10.1093/ajcn/82.1.222S
72. Laaksonen D.E. еt al. Weight loss and weight maintenance, ambulatory blood pressure and cardiac autonomic tone in obese persons with the metabolic syndrome. J. Hypertens. 2003. 21. 371-378. doi: 10.1097/00004872-200302000-00029
73. Straznicky N.E. et al. The effects of weight loss versus weight loss maintenance on sympathetic nervous system activity and metabolic syndrome components. J. Clin. Endocrinol. Metab. 2011. 96. E503-E508. doi: 10.1210/jc.2010-2204
74. Gadde K.М., Martin C.К., Berthoud H.R., Heymsfield S.B. Obesity: pathophysiology and management. J. Am. Coll. Cardiol. 2018. 71. 69-84. doi: 10.1016/j.jacc.2017.11.011
75. Siebenhofer A. et al. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database Syst. Rev. 2016. 3. CD007654. doi: 10.1002/14651858.CD007654.pub4
76. Cohen J.В., Gadde K.M. Weight loss medications in the treatment of obesity and hypertension. Curr. Hypertens. Rep. 2019. 21. 16. doi: 10.1007/s11906-019-0915-1
77. Wilding J.Р.Н. et al. STEP 1 Study Group. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 2021. 384. 989. doi: 10.1056/NEJMoa2032183
78. FDA Drug Safety Communication. Safety clinical trial shows possible increased risk of cancer with weight-loss medicine Belviq, Belviq XR (lorcaserin) issued on January 14, 2020. Accessed July 26, 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawalweight-loss-drug-belviq-belviq-xr-lorcaserin-market
79. Zhang S., Manne S., Lin J., Yang J. Characteristics of patients potentially eligible for pharmacotherapy for weight loss in primary care practice in the United States. Obes. Sci. Pract. 2016. 2. 104-114. doi: 10.1002/osp4.46
80. English W.J. et al. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg. Obes. Relat. Dis. 2018. 14. 259-263. doi: 10.1016/j.soard.2017.12.013
81. Ponce J. et al. American Society for Metabolic and Bariatric Surgery estimation of bariatric surgery procedures in 2015 and surgeon workforce in the United States. Surg. Obes. Relat. Dis. 2016. 12. 1637-1639. doi: 10.1016/j.soard.2016.08.488
82. Mechanick J.I. et al.; American Association of Clinical Endocrinologists; Obesity Society; American Society for Metabolic & Bariatric Surgery. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient — 2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring). 2013. 21(suppl. 1). S1-S27. doi: 10.1002/oby.20461
83. Rubino F. et al.; on behalf of the Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016. 39. 861-877. doi: 10.2337/dc16-0236
84. Sandoval D.А., D’Alessio D.A. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol. Rev. 2015. 95. 513-548. doi: 10.1152/physrev.00013.2014
85. Bonfils P.К. et al. Roux-en-Y gastric bypass alleviates hypertension and is associated with an increase in mid-regional pro-atrial natriuretic peptide in morbid obese patients. J. Hypertens. 2015. 33. 1215-1225. doi: 10.1097/HJH.0000000000000526
86. Fenske W.К. et al. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: a 12-month prospective study. Surg. Obes. Relat. Dis. 2013. 9. 559-568. doi: 10.1016/j.soard.2012.03.009
87. Buchwald H. et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004. 292. 1724-1737. doi: 10.1001/jama.292.14.1724
88. Hallersund P. et al. Gastric bypass surgery is followed by lowered blood pressure and increased diuresis: long term results from the Swedish Obese Subjects (SOS) study. PLoS One. 2012. 7. e49696. doi: 10.1371/journal.pone.0049696
89. Adams T.D. et al. Weight and metabolic outcomes 12 years after gastric bypass. N. Engl. J. Med. 2017. 377. 1143-1155. doi: 10.1056/NEJMoa1700459
90. Schauer P.R. et al.; STAMPEDE Investigators. Bariatric Surgery versus intensive medical therapy for diabetes: 5-year outcomes. N. Engl. J. Med. 2017. 376. 641-651. doi: 10.1056/NEJMoa1600869
91. Schiavon C.А. et al. Effects of bariatric surgery in obese patients with hypertension: the GATEWAY randomized trial (Gastric Bypass to Treat Obese Patients With Steady Hypertension). Circulation. 2018. 137. 1132-1142. doi: 10.1161/CIRCULATIONAHA.117.032130
92. Schiavon C.А. et al. Three-year outcomes of bariatric surgery in patients with obesity and hypertension: a randomized clinical trial. Ann. Intern. Med. 2020. 173. 685-693. doi: 10.7326/M19-3781
93. Khan S. et al. Trends in bariatric surgery from 2008 to 2012. Am. J. Surg. 2016. 211. 1041-1046. doi: 10.1016/j.amjsurg.2015.10.012
94. Chang S.Н. et al. The effectiveness and risks of bariatric surgery: an updated systematic review and metaanalysis, 2003–2012. JAMA Surg. 2014. 149. 275-287. doi: 10.1001/jamasurg.2013.3654
95. Aminian A. et al. How safe is metabolic/ diabetes surgery? Diabetes Obes. Metab. 2015. 17. 198-201. doi: 10.1111/dom.12405
96. Hales C.М., Carroll M.D., Fryar C.D., Ogden C.L. Pre-valence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017. 1-8.
97. Daniels S. et al. American Heart Association Childhood Obesity Research Summit: executive summary. Circulation. 2009. 119. 2114-2123. doi: 10.1161/CIRCULATIONAHA.109.192215
98. Miliku K. et al. Associations of maternal and paternal blood pressure patterns and hypertensive disorders during pregnancy with childhood blood pressure. J. Am. Heart Assoc. 2016. 5. e003884. doi: 10.1161/JAHA.116.003884
99. Brown M.А. et al.; International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management re-commendations for international practice. Hypertension. 2018. 72. 24-43. doi: 10.1161/HYPERTENSIONAHA.117.10803
/18.jpg)

/22.jpg)
/25.jpg)