The article was published on p. 61-66
Introduction
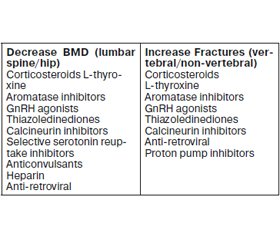
Osteoporosis is a metabolic bone disease characterized by a deterioration of bone microarchitecture which conduces to fragility fractures. It is a heterogeneous and multifactorial etiological disease. About 20 to 30 % of osteoporotic postmenopausal women and more than 50 % men have a secon–dary induced etiology, namely the medications (Table 1).
The actual increase in human longevity is possible due to the public health care and to polimedication, mainly of the oldest people. Unfortunately, some of the most prescribed drugs to chronic diseases may have negative effects on the skeleton, not only decreasing bone mass, but also increasing the risk for fragility fractures.
In the recent years, the list of such drugs and their potential mechanisms for reducing bone mass and bone quality and/or of inducing fractures, is becoming bigger, exceeding the classic glucocorticoids actions in the bone tissue.
More, the population with multiple and chronic medications has already an increased risk of osteoporosis and fractures. So, the clinicians must be aware and the potential risks and benefits of such medications should be balanced.
Corticosteroids
Glucocorticoids are the main drug-induced bone loss and secondary cause of osteoporosis; their mechanism of action includes primarily the reduction in bone formation, as well as the increase in bone resorption, induction of hypogonadism, decrease in calcium absorption with augmen–ted parathyroid hormone (PTH) and increase in calcium excretion [1]. More recent data suggest that the glucocorticoid-induced metabolic disruption, which is responsible for central obesity, diabetes mellitus, hyperlipidemia and insulin resistance are mediated by the inhibition of osteocalcin secretion in the osteoblasts; however it does not explain the decrease in bone formation rate and low bone mass. The bone loss is predominantly at the axial skeleton and bone mineral density (BMD) can decrease between 5 and 15 % per year, being more pronounced in the first 3 to 12 months. The fragility fractures risk is increased in the first 3 to 6 months after the beginning of the chronic therapy reaching about 30 to 50 % [2].
The use of inhaled corticosteroids especially in mode–rate to high doses, can also induce bone loss (although less than oral and intra-articular use) and those patients should always be evaluated mainly if they have other risk factors for osteoporosis and fragility fractures and/or falls [3].
The myopathy induced by these drugs can also contri–bute to increase the falls frequency and consequently to the osteoporotic fractures.
Some factors which increase the risk for the fragility fractures in patients on glucocorticoids have been identified: reduced body mass index, patients older than 60 years, pre–valent fracture(s), diseases that need corticosteroid treatment and actual and cumulative dose of corticosteroid (above 5 mg/day of oral prednisone or equivalent for more than 3 months).
Usually, the anti-osteoporotic treatment is indicated if one or more of the above risk factors are present and the patient will be on corticosteroids for 3 months or more with a daily prednisone or equivalent dose > 7.5 mg. It is controversial to treat women with childbearing potential.
All patients should receive calcium and vitamin D supplements. Assessing the baseline fracture risk and incorporate the dose and anticipated duration of glucocorticoid therapy into treatment decisions are in the prevention and treatment of glucocorticoid-induced osteoporosis of the American College of Rheumatology recommendations. The preferred drugs for treatment are the bisphosphonates (oral and intravenous) and more recently, teriparatide and denosumab [1].
Sex steroid hormone inhibitors
Drugs causing hypogonadism such as aromatase inhibitors and GnRH agonists are one of the main causes of bone mass loss and fractures. Hypogonadism is the most important cause of osteoporosis in men, occurring in up to 20 % of men with symptomatic vertebral fractures and 50 % of old men with hip fractures [4].
The hormonal therapy with aromatase inhibitors and with GnRH agonists is based in originating an estrogen depletion state; consequently, a down-regulation of osteoprotegerin expression occurs, causing increased osteoclastic bone resorption, which leads to decreased bone mass.
Aromatase inhibitors
The aromatase inhibitors (anastrozole, letrozole, exe–mestane) are used in postmenopausal women with positive estrogen-receptor breast tumors. These medications inhibit the enzyme aromatase, which aromatises the androgens produced in the adrenal glands to estrogens, originates approximately 95–99 % reduction in the circulating estradiol levels. This effect is beneficial to control the disease, but originates about 2 %/year bone mass loss (double of the normal postmenopausal), low BMD and osteoporosis.
The disease and also other treatments like surgery, chemotherapy and radiotherapy may contribute to the bone mass loss; thus, these women have a higher risk for fragility fractures, especially at the lumbar spine.
The ATAC study showed that the anastrozole group alone (versus the anastrozole and tamoxifen combined group) had an augmented incidence of osteoporotic fractures and decreases in baseline BMD (from 6.98 % at the lumbar spine and 7.24 % at the hip after 5 years [5].
Oral bisphosphonates, zoledronate and denosumab may prevent the aromatase inhibitors-related bone loss.
GnRH agonists
GnRH (or luteinizing hormone- releasing hormone) agonists (leuprolide and goserelin) are widely used in the treatment of hormone-dependent cancers therapy such as the prostate and the breast, acting through the inhibition of the pituitary gonadotropins.
In premenopausal women with estrogen-receptor positive breast tumors, these medications are used to inhibit estrogens production, inducing hypogonadism. The reduction in circulating estrogens can reach 98 % and BMD decreases from 6 to 10 % in the first 2 years, with a recovery after drug discontinuation. The data regarding osteoporotic fractures are scarce and it does not seem to be increased in women with normal BMD.
In prostate cancer the aim of this therapy is to inhibit the testosterone production, which is a very important hormone maintaining bone health, mainly at the bone formation. However, a secondary decrease in estrogens is also detected and appears to be primarily responsible for the skeletal adverse effects of these drugs. An increase in PTH-mediated osteoclast activation can contribute to the physiopathology of the bone loss. In the first year of therapy, men on these drugs have a maximal «acute» loss of bone mass; in the first year of therapy, the bone loss rate is about 2–8 % at the lumbar spine and 1.8–6.5 % at the hip. The BMD decrease at the distal radius is about 5.3 % in 12 months. The occurrence of fractures is also increased and is correlated with the duration of the treatment and it has been described that patients treated during with at least 9 doses of these drugs are more prone to fractures in the first year.
The efficacy of both oral and intravenous bisphosphonates (pamidronate and zoledronate) as well the selective estrogen-receptor modulatos (raloxifene, toremifene) and denosumab has been shown in men with non-metastatic prostate cancer, preventing bone loss and inducing increases in BMD, thus reducing the fracture risk [6–9].
Thiazoledinediones (Glitazones)
Thiazoledinediones used in type 2 diabetic patients have major insulin-sensitizing effects in the liver, in the muscle and in the adipose tissue through the activation of the pero–xisome proliferator-activated receptor gamma. However, their ability to deviate the differentiation of the stem cells from osteoblasts to adipocytes increases the adipogenesis and reduces the osteoblastogenesis, thus reducing the bone formation. So, the previous studies showed an association of these drugs to the BMD reduction in both lumbar spine and hip and osteoporotic fractures, mainly in women with type 2 diabetes mellitus.
Rosiglitazone and pioglitazone have been associated to an increase in the incidence of fragility fractures at the humerus, hands and feet; the risk seems to be higher in postmenopausal women and is also dose-dependent. However, more recent studies suggest that the risk of osteoporotic fractures is similar in patients using thiazoledinediones or insulin, and so, it remains unclear if the association between these medications and fracture risk is related to the drug or to the diabetes itself [10].
Serotonin selective reuptake inhibitors
The central nervous system has a recognized serotonin signaling: serotonin is synthesized in presynaptic neurons, accumulates in vesicles and stimulates the postsynaptic neurons through its receptor. Inhibition of this pathway prevents uptake of serotonin, resulting in its accumulation within the synaptic cleft and the prolonging of receptor activation.
Since some years ago the serotoninergic system is known to play a role in bone metabolism, through functional serotoninergic pathways in bone cells; indeed, osteoblasts, osteoclasts and osteocytes express the serotonin receptors and serotonin selective reuptake inhibitors (SSRI) inhibit serotonin uptake in bone cells in the same manner as in neurons [11, 12].
SSRI like citalopram, escitalopram, fluoxetine, paro–xetine, sertraline, fluvoxamine, seem to originate bone mass loss and even change bone architecture. In a prospective study during 5 years, 5,008 patients taking serotonin selective reuptake inhibitors had a lower BMD at both hip and spine, increased falls and increased risk of clinical fragility fractures. Clinical longitudinal studies found about a 1.6-fold of greater decline in BMD in the SSRI users vs non-users. However, the Women’s Health Initiate and the National Health and Nutrition Survey did not find association with a decrease in BMD [13].
Regarding osteoporotic fractures, some studies like MrOS and CaMOS from Canada showed, respectively, an increase in the risk of non-spine fractures and higher 5-years fractures rates for the SSRI users [12, 14].
Also regarding the classic tricyclic antidepressants, a meta-analysis showed they confer an increased risk of fragility fractures, independent of depression and BMD [15].
So, it is far from clarified the relative importance of the major depressive disease and its co-morbidities like reduced food ingestion, low body weight, hypogonadism, and/or its treatment in the bone mass loss and in the fractures observed in these patients [16].
Second-generation antipsychotics
The second-generation antipsychotics like olanzapine, clozapine and risperidone are actually very used in clinical practice not only in adults with bipolar disorders and schizophrenia, but even in children with problems of irritability and autism. Their negative effect on insulin sensitivity is responsible for their induction of obesity, type 2 diabetes and dyslipidemia. Also in bone, negative effects have been found, namely reduced bone mineral density and increased fracture rate. Some studies showed that risperidone can cause hyperprolactinemia and secondary hypogonadism due to the blockade of dopamine receptors and that could be one of the mechanisms for bone loos. However, it is known that second-generation anti-psychotics have multiple targets like the serotonin, D2 and histamine receptors, and so can indirectly and through many pathways influence the bone remodeling. Experimental studies on mice suggest that risperidone affects both bone formation and resorption, decreasing bone mass and changing bone microarchitecture. This is a very important issue mainly in children and adolescents, because there are still building their skeleton and acquiring the peak bone mass. Also, studies in humans are needed to clarify all these issues [17].
L-thyroxine
The thyroid hormone is essential for the normal skeletal development and linear growth, so both deficiency and excess have negative skeletal consequences; also in adults the excess of thyroid hormones are detrimental for the skeleton because they increase bone turnover, shortening the bone remodeling cycle and causing up to 10 % loss of mineralized bone per cycle. More recently, studies revealed that thyroid stimulating hormone (TSH) alone is able to modulate the bone turnover; thus, when it is suppressed, the bone resorption also increases [18, 19].
The thyroid hormone (L-thyroxine) is usually prescribed in the treatment of hypothyroidism with a replacement dose; however, in thyroidectomized patients due to thyroid carcinoma, a higher dose is used in order to suppress the plasma TSH concentrations originating a subclinical hyperthyro-idism. Thyroid hormone increases directly the bone resorption and indirectly by stimulating bone-resorbing cytokines decreasing the BMD predominantly at the cortical bone.
Some studies have shown that patients aged 70 or more years on chronic L-thyroxine therapy had a significantly higher risk for osteoporotic fractures with a strong dose-response relation, as the overtreatment results in very low TSH levels [20].
We have also showed that young men with endogenous hyperthyroidism have an increase in the prevalence of reduced bone mass and osteoporosis and even asymptomatic vertebral fractures detected by VFA (Vertebral Fracture Assessment) [21, 22].
Proton pump inhibitors
Some studies in rats have shown that long-term administration of proton pump inhibitors like omeprazole originates reduced bone density. Also in humans using omeprazole, decreases in serum and urinary calcium levels were documen-ted, because the gastric acid is important for the absorption of calcium supplements, especially the calcium carbonate.
Moreover, the protein content of the meal influences the calcium absorption. Some case-control studies in humans have shown an association between gastric acid suppression and fractures, with significant increase in hip fractures risk. The association was also dependent on duration of the proton pump inhibitors use and their different effect in men and women seemed to depend on calcium intake.
It seems that the use of a high dose more than one year can increase the risk of bone fractures in 10–40 %, especially hip and spine and that risk could reverse after one year of discontinuation [23, 24].
Also, studies with H2 receptor antagonists revealed conflicting results regarding the fracture risk.
So, at this time, the association of these drugs to reduced BMD and to fragility fractures is not completely clarified and more studies are needed to confirm the real negative skeletal effects of the proton pump inhibitors [25–28].
Antiretroviral drugs
Acquired immune deficiency syndrome (AIDS) itself is associated to low BMD and fragility fractures, which have a multifactorial etiology, namely chronic immune activation and upregulation of pro-reasorptive cytokines (tumor necrosis factor alfa, interleukin-6, receptor activator of nuclear factor kappa-B ligand), direct effects of human immunodeficiency virus 1 (HIV-1) viral proteins on bone cells, low body weight and hepatitis C coinfection [29, 30].
Moreover, the highly active treatments for AIDS have significantly decreased both morbidity and mortality associated with the disease; however, some side effects emerged, namely the bone disease. The prevalence of osteoporosis in men and premenopausal women HIV infected is already increased and is about 3 times higher than the non-infected population [31, 32].
The AIDS patients on antiretroviral drugs (both protease and reverse-transcriptase inhibitors) have higher prevalence of low BMD and osteoporosis. Within the first 1–2 years after initiation the anti-retroviral therapy, there is about 2–4 % loss BMD, both at the lumbar spine or hip detected by dualenergy x-ray absorptiometry (DXA). The peak bone loss occurs in the first 6 and 12 months, respectively, at the lumbar spine and at the hip. All the antiretrovirals and in different regimens seem to be similar, although most studies agree that tenofovir induces a more pronounced bone loss [33].
Despite the pathogenesis being controversial, the increased osteoclastogenesis seems to be an important mechanism originating an increase in the bone resorption. Furthermore, the impaired osteoblastic function through the Wnt signaling contributes to reduce the BMD. Also direct effects on vitamin D metabolism seem to be implicated [34, 35].
Loop diuretics
These drugs, like furosemide, often used in the treatment of congestive heart failure, increase renal calcium excretion and originate an increased bone turnover, with reduced BMD and risk of fragility fractures [36].
Anti-epileptics
These drugs have been associated to BMD reduction, changes in bone quality, osteoporosis and fractures. The pathophysiology includes various mechanisms, namely the induction of the liver cytochrome P450, increasing the catabolism of vitamin D into inactive metabolites and thus, se-condary hyperparathyroidism and increase in PTH-mediated bone resorption. Also, the direct action in the osteoblasts, the changes in calcium absorption, the increase in homocysteine and the reduction in vitamin K and in sex steroids, are possible mechanisms implicated in the bone loss. The drugs that have been studied and associated to the bone disease are the phenytoine, the phenobarbital and the carbamazepine. Valproate, despite being an inhibitor of the liver cytochrome P450, can reduce the BMD at several skeletal sites [37–39].
Anticoagulants
The unfractionated heparin can both activate the osteoclasts because it binds to osteoprotegerin, and also inhibit the differentiation and function of the osteoblasts decreasing bone formation. About one third of patients on heparin can have reduced BMD, however fractures are rare. By opposite, the patients on low-weight heparin have even less fractures [40, 41].
Oral anticoagulants, like warfarin, antagonize the vitamin K and inhibits the gamma-carboxylation of osteocalcin. Despite that, the patients with reduced BMD and fractures are not so evident [42].
The new generation anticoagulants like dabigatran and rivaroxaban do not seem to be associated to bone disease. However, studies in vitro with rivaroxaban show that it may reduce the function of the osteoblasts [43, 44].
Imunossupressants
The patients undergoing transplantation are prone to bone mass loss and fractures relatively frequently. That is usually due to the use of imunossupressants like calcineurin inhibitors, namely tacrolimus and cyclosporine A; they can originate bone mass loss and osteoporosis, but its mechanism is still debated. In the period immediately after the transplantation, the patients are particularly prone to fractures: in the liver, heart and lung transplantation, the spine and the ribs fragility fractures are more frequent, while in the kidney transplantation the long bones and feet fractures are the most frequent [45–47].
Other drugs like somatostatin analogs can decrease the vitamin D absorption and insulin-like growth factor 1 levels, while oral retinoids can activate the osteoclasts.
Conclusions
The mechanisms of action and the effects on BMD and fractures, for some of these medications, have been well defined, while for other drugs more studies are still needed for a better evaluation of their impact on the bone. The table 2 summarizes some drug effects on the BMD and fractures.
In all the patients with such medications, it is very important to monitor the bone mass by DXA and/or with the bone remodeling markers. It is also very important to use the less efficacious dose. The individuals should be also advised to modify the lifestyle to protect bone mass, such as regular exercise, adequate sun exposure, stop smoking, decrease in alcohol consumption and an adequate calcium and vitamin D ingestion (above 50 years-old in women and 70 years-old in men 1,200 mg and 800–1,000 IU daily, respectively). The intervention strategies to reduce falls should be also encouraged. Sometimes, the anti-resorptive and/or bone-formation anti-osteoporotic agents are also needed (Table 3) [48, 49].
Список литературы
1. Canalis E., Mazziotti G, Bilezikian J.P. Glucocorticoid-induced osteoporosis: pathophysiology and therapy // Osteoporos. Int. — 2007. — Vol. 18. — P. 1319-1328.
2. Manolagas S.C. Corticosteroids and fractures: a close encounter of the third cell kind // J. Bone Miner. Res. — 2000. — Vol. 15. — P. 1001-1005.
3. Chee C., Sellahewa L., Pappachan J.M. Inhaled corticosteroids and bone health // The Open Respiratory Medicine Journal. — 2014. — Vol. 8, Suppl. 1. — P. 85-92.
4. Tuck S.P., Francis R.M. Testosterone, bone and osteoporosis // Frontiers of Horm. Res. — 2009. — Vol. 37. — P. 123-132.
5. Eastell R., Hannon R.A., Cuzick J. et al. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrazole, Tamoxifen, Alone or in Combination (ATAC) trial // J. Bone Min. Res. — 2006. — Vol. 21. — P. 1215-1223.
6. Greenspan S.L. Approach to the prostate cancer patient with bone disease // J. Clin. Endocrinol. Metab. — 2008. — Vol. 93. — P. 2-7.
7. Shahinian V.B., Kuo Y.F., Freeman J.L. et al. Risk of fracture after androgen deprivation for prostate cancer // N. Engl. J. Med. — 2005. — Vol. 352. — P. 154-164.
8. Shahinian V.B., Kuo Y., Freeman J.L. et al. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate cancer // Cancer. — 2005. — Vol. 103. — P. 1615-1624.
9. Mittan D., Lee S., Miller E. et al. Bone loss following hypogonadism in men with prostate cancer treated with GnRH analogs // J. Clin. Endocrinol. Metab. — 2002. — Vol. 87. — P. 3656-3661.
10. Bazelier M.T., Vestergaard P., Gallagher A.M. et al. Risk of fracture with thiazoledinediones: disease or drugs? // Calcif. Tissue Int. — 2012. — Vol. 90. — P. 450-457.
11. Warden S.J., Robling A.G., Haney E.M. et al. The emer–ging role of serotonin (5-hydroxytryptamine) in the skeleton and its mediation of the skeletal effects of low-density lipoprotein receptor-related protein 5 (LRP5) // Bone. — 2010. — Vol. 46. — P. 4-12.
12. Bliziotes M. Update in serotonin and bone // J. Clin. Endocrinol. Metab. — 2010. — Vol. 95. — P. 4124-4132.
13. Spangler L., Scholes D., Brunner R.L. et al. Depressive symptoms, bone loss and fractures in postmenopausal women // J. Gen. Intern. Med. — 2008. — Vol. 23. — P. 567-574.
14. Chau K., Atkinson S., Taylor V. Are selective serotonin reuptake inhibitors a secondary cause of low bone density? // J. Osteop. — 2012.
15. Wu Q., Wenchun Q., Crowell M.D. et al. Tricyclic antidepressant use and risk of fractures: a meta-analysis of cohort and case-control studies // J. Bone Min. Res. — 2013. — Vol. 28. — P. 753-762.
16. Haney E.M., Warden S.J., Bliziotes M.M. The effects of selective serotonin reuptake inhibitors on bone health in adults: time for recommendations about screening // Bone. — 2010. — Vol. 46. — P. 13-17.
17. Motyl K.J., Dick-de-Paula I., Maloney A.E. et al. Trabecular bone loss after administration of the second-generation antipsychotic risperidone is independent of weight gain // Bone. — 2012. — Vol. 50. — P. 490-498.
18. Bassett J.H., Williams G.R. The molecular actions of thyroid hormone in bone // Trends in Endocrinol. and Metab. — 2003. — Vol. 14. — P. 356-364.
19. Abe E., Marians R.C., Yu W. et al. TSH is a negative regulator of skeletal remodeling // Cell. — 2003. — Vol. 115. — P. 151-162.
20. Turner M., Camacho X., Fischer H. et al. Levothyro-xine dose and risk of fractures in older adults: nested case-control study // Br. Med. J. — 2011. — Vol. 342. — P. 1-9.
21. Barbosa A.P., Mascarenhas M.R., Simões V. et al. Subcli–nical and overt hyperthyroidism effect on BMD and soft tissue composition of elderly women // J. Bone Min. Res. — 2012. — S. 427.
22. Barbosa A.P., Mascarenhas M.R., Silva C.F. et al. Prevalence of silent vertebral fractures detected by vertebral fracture assessment in young Portuguese men with hyperthyroidism // Eur. J. Endocrinol. — 2015. — Vol. 172. — P. 189-194.
23. Roux C., Briot K., Gossec L. et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole // Calcif. Tissue Int. — 2009. — Vol. 84. — P. 13-19.
24. Yu E.W., Blackwell T., Ensrud K.E. et al. Acid-Suppressive medications and risk of bone loss and fractures in older adults // Calcif. Tissue Int. — 2008. — Vol. 83. — P. 251-259.
25. Solomon D.H., Diem S. J., Ruppert K. et al. Bone mi–neral density changes among women initiating proton pump inhibitors or H2 receptor antagonists: a SWAN cohort study // J. Bone Min. Res. — 2014. — Vol. 30. — P. 232-239.
26. Vestergaard P. Proton pump inhibitors, histamine H2 receptor antagonists and other antacid medications and the risk of fracture // Calc. Tissue Int. — 2006. — Vol. 79. — P. 76-83.
27. Yang Y., Lewis J.D., Epstein S. et al. Long-term proton pump inhibitor therapy and risk of hip fracture // JAMA. — 2006. — Vol. 296. — P. 2947-2953.
28. Yu E.W., Bauer S.R., Bain P.A. et al. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international stu–dies // Am. J. Med. — 2011. — Vol. 124. — P. 519-526.
29. Hileman C.O., Labbato D.E., Storer N.U. et al. Is bone loss linked to chronic inflammation in antiretroviral-naïve HIV-infec–ted adults? // AIDS. — 2014. — Vol. 28. — P. 1759-1767.
30. Cotter E.J., Chow N., Powderly W.G. et al. HIV type 1 alters mesenchymal stem cell differentiation potential and cell phenotype ex vivo // AIDS Res. Hum. Retroviruses. — 2011. — Vol. 150. — P. 67-78.
31. Shiau S., Broun E.C., Arpadi S.M. et al. Incident fractures in HIV-infected individuals: a systematic review and meta-analysis // AIDS. — 2013. — Vol. 27. — P. 1949-1957.
32. Bedimo R., Maalouf N.M., Zhang S. et al. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents // AIDS. — 2012. — Vol. 26. — P. 825-831.
33. Grigsby I.F., Pham L., Mansky L.M. et al. Tenofovir treatment of primary osteoblasts alters gene expression profiles: implications for bone mineral density loss // Biochem. Biophys. Res. Commun. — 2010. — Vol. 394. — P. 48-53.
34. Malizia A.P., Cotter E., Chew N. et al. HIV protease inhibitors selectively induce gene expression alterations associated with reduced calcium deposition in primary human osteoblasts // AIDS Res. Hum. Retroviruses. — 2007. — Vol. 23. — P. 243-250.
35. Brown T.T., McComsey G.A. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D // Antivir. Ther. — 2010. — Vol. 15. — P. 425-429.
36. Carbone L.D., Johnson K.C., Bush A.J. et al. Loop diuretic use and fracture in postmenopausal women: findings from the Wo–men’s Health Initiative // Arch. Int. Med. — 2009. — Vol. 169. — P. 132-140.
37. Vestergaard P. Epilepsy, osteoporosis and fracture risk — a meta-analysis // Acta Neurol. Scand. — 2005. — Vol. 112. — P. 277-286.
38. Omdahl J.L., Morris H.A., May B.K. Hydroxilase enzymes of the vitamin D pathway: expression, function and regulation // Ann. Rev. Nutr. — 2002. — Vol. 22. — P. 139-166.
39. Fitzpatrick L.A. Pathophysiology of bone loss in patients receiving anticonvulsant therapy // Epilepsy Behav. — 2004. — Vol. 5. — S. 3-15.
40. Muir J.M., Andrew M., Hirsh J. et al. Histomorphometric analysis of the effects of standard heparin on trabecular bone in vivo // Blood. — 1996. — Vol. 88. — P. 1314-1320.
41. Irie A., Takami M., Kubo H. et al. Heparin enhances osteoclastic bone resorption by inhibiting osteoprotegerin activity // Bone. — 2007. — Vol. 41. — P. 165-174.
42. Jamal S.A., Browner W.S., Bauer D.C. et al. Warfarin use and risk for osteoporosis in elderly women. Study of Osteoporotic Fractures Research Group // Ann. Int. Med. — 1998. — Vol. 128. — P. 829-832.
43. Fusaro M., Carbonare L.D., Dusso A. et al. Differential effects of dabigatran and warfarin on bone volume and structure in rats with normal renal function // PLoS One. — 2015. — Vol. 10.
44. Solayar G.N., Walsh P.M., Mulhall K.J. The effect of a new direct factor Xa inhibitor on human osteoblasts: an in vitro study comparing the effect of rivaroxaban with enoxaparin // BMC Musculoskelet. Disord. — 2011. — Vol. 12. — P. 247.
45. Maalouf N.M., Shane E. Osteoporosis after solid organ transplantation // J. Clin. Endocrinol. Metab. — 2005. — Vol. 90. — P. 2456-2465.
46. Epstein S., Shane E., Bilezikian J.P. Organ transplantation and osteoporosis // Curr. Opin. Rheumatol. — 1995. — Vol. 7. — P. 255-261.
47. Zhang R., Chouhan K.K. Metabolic bone diseases in kidney transplant recipients // World J. Nephrol. — 2012. — Vol. 1. — P. 127-133.
48. Pitts C., Kearns A. Update on medications with adverse ske–letal effects // Mayo Clin. Proc. — 2011. — Vol. 86. — P. 338-343.
49. Mazziotti G., Canalis E., Giustina A. Drug-induced osteoporosis: mechanisms and clinical implications // Am. J. Med. — 2010. — Vol. 123. — P. 877-884.
/61.jpg)
/64.jpg)
/65.jpg)