Журнал "Гастроэнтерология" Том 55, №3, 2021
Вернуться к номеру
Катастрофічний антифосфоліпідний синдром (синдром Ашерсона), обумовлений ймовірною нейроендокринною пухлиною кишечника: випадок із практики
Авторы: V.P. Shypulіn, V.V. Cherniavskyi, L.S. Hvozdecka, A.V. Neverovskyi, V.V. Tishchenko
Bogomolets National Medical University, Kyiv, Ukraine
Рубрики: Гастроэнтерология
Разделы: Справочник специалиста
Версия для печати
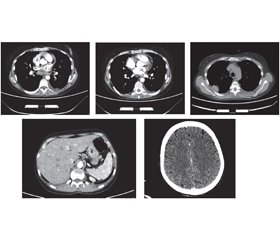
Актуальність. Щороку в усьому світі кількість людей із рідкісними формами хвороб збільшується. Однією з них є катастрофічний антифосфоліпідний синдром — синдром Ашерсона. На сьогодні він активно досліджується, однак патофізіологічні механізми його розвитку ще не вивчені до кінця. Наша робота є першою спробою опису синдрому Ашерсона на прикладі клінічного випадку в Україні. Мета: визначення факторів і механізмів, що призвели до смертi пацієнта з синдромом Ашерсона в Україні. Матеріали та методи. Під час роботи використані структурно-логічний аналіз і клініко-статистичний метод. Результати. На основі комплексу клінічних і лабораторних критеріїв наша медична група поставила пацієнту клінічний діагноз системного червоного вовчака, ускладненого катастрофічним антифосфоліпідним синдромом. Незважаючи на лікування глюкокортикоїдами та антикоагулянтами відповідно до міжнародних рекомендацій, пацієнт помер. Результати розтину показали, що безпосередньою причиною смерті став великий тромб, який закупорював легеневу артерію та її основні гілки. При гістологічному дослідженні було виявлено тромбоз дрібних судин нирок і мозку, підозрювали нейроендокринну пухлину (G2; pT3pNxpM1b) тонкої кишки з метастазами в печінку, мозок, міокард та нирки. Для уточнення гістологічного діагнозу було проведене додаткове імуногістохімічне дослідження. Морфологічна картина і результати імуногістохімічного дослідження найбільше відповідають помірно диференційованому (G2) незроговілому плоскоклітинному раку (код МКХ-О: 8070/3) із пошкодженням стінок тонкої кишки, печінки, легенів, нирок, міокарда та мозку. Висновки. Наведені дані підкреслюють, що, незважаючи на рідкість синдрому Ашерсона, завжди необхідно враховувати його ймовірність у разі ознак множинного тромбозу та поліорганної недостатності. Оскільки його розвиток є наслідком серйозних захворювань, серед яких захворювання сполучної тканини, злоякісні утворення, інфекції, етіотропне та патогенетичне лікування може запобігти розвитку катастрофічного антифосфоліпідного синдрому та смерті.
Background. Every year, the number of people with rare forms of the disease is increasing worldwide. One of these is the catastrophic antiphospholipid syndrome — Asherson’s syndrome. To date, it is being actively studied, but the pathophysiological mechanisms of its development have not yet been fully investigated. Our work is the first attempt to describe Asherson’s syndrome on the example of a clinical case in Ukraine. Objective: to determine the factors and mechanisms that led to the death of a patient with Asherson’s syndrome in Ukraine. Materials and methods. The structural-logical analysis and the clinical-statistical method were used. Results. Based on clinical and laboratory criteria, our medical team established a clinical diagnosis of systemic lupus erythematosus complicated by a catastrophic antiphospholipid syndrome (CAPS). Despite treatment with glucocorticoids and anticoagulants according to international guidelines, the patient died. The autopsy results showed that the immediate cause of death was a large blood clot that blocked the pulmonary artery and its main branches. Histological examination revealed thrombosis of small vessels of the kidneys and brain; a neuroendocrine tumor (G2; pT3pNxpM1b) of the small intestine with metastases to the liver, brain, myocardium and kidneys was suspected. An additional immunohistochemical study was performed to clarify the histological diagnosis. The morphological picture and results of immunohistochemical study mostly correspond to the moderately differentiated (G2) non-keratinizing squamous cell carcinoma (ICD-O code: 8070/3) with damage to the walls of the small intestine, liver, lungs, kidneys, myocardium and brain. Conclusions. These data emphasize that despite the rarity of Asherson’s syndrome, it is always necessary to consider its probability in the presence of signs of multiple thrombosis and multiple organ failure. Because its development is the result of serious diseases, including connective tissue diseases, malignancies, infections, the etiotropic and pathogenetic treatment can prevent the development of CAPS and death.
катастрофічний антифосфоліпідний синдром; системний червоний вовчак; плоскоклітинний рак
catastrophic antiphospholipid syndrome; systemic lupus erythematosus; squamous cell carcinoma
Introduction
Case report
Discussion
Conclusions
- Asherson R.A. The catastrophic antiphospholipid syndrome. J. Rheumatol. 1992. 19(4). 508-12.
- Cervera R., Piette J.C., Font J. et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of di-sease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002. 46. 1019-27.
- Bucciarelli S., Espinosa G., Cervera R. et al. Mortality in the catastrophic antiphospholipid syndrome: causes of death and prognostic factors in a series of 250 patients. Arthritis Rheum. 2006. 54. 2568-2576.
- Cervera R. CAPS Registry. Lupus. 2012. 21(7). 755-7.
- Rodríguez-Pintó I., Shoenfeld Y., Erkan D., Garriga R.E., Cervera R. Catastrophic antiphospholipid syndrome (CAPS). Descriptive analysis of 500 patients from the International CAPS Registry. Lupus. 2016. 25. 12-3.
- Miesbach W. Malignancies and catastrophic anti-phospholi-pid syndrome. Clinic Rev. Allerg. Immunol. 2009. 36. 91-97.
- Petri M., Orbai A.M., Alarcón G.S. et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012. 64(8). 2677-86.
- Asherson R.A., Cervera R., de Groot P. et al. Catastrophic antiphospholipid syndrome: International consensus statement on classification criteria and treatment guidelines. Lupus. 2003. 12. 530-534.
- Cervera R., Font J., Gómez-Puerta J.A. et al. Validation of the preliminary criteria for the classification of catastrophic antiphospholipid syndrome. Ann. Rheum. Dis. 2005. 64. 1205-1209.
- Legault K., Schunemann H., Hillis C. et al. McMaster RARE-Bestpractices clinical practice guideline on diagnosis and management of the catastrophic antiphospholipid syndrome. J. Thromb. Haemost. 2018. 16(8). 1656-1664.
- Carmi O., Berla M., Shoenfeld Y., Levy Y. Diagnosis and management of catastrophic antiphospholipid syndrome. Expert Rev. Hematol. 2017 Apr. 10(4). 365-374.
- Konstantinides S.V., Torbicki A., Agnelli G. et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014. 35(43). 3033-69, 3069a-3069k.
- Kitchens C.S. Thrombotic storm: when thrombosis begets thrombosis. Am. J. Med. 1998. 104. 381-385.
- Asherson R.A. Antiphospholipid antibodies, malignancies and paraproteinemias. J. Autoimmun. 2000 Sep. 15(2). 117-22.
- Sciascia S., Lopez-Pedrera C., Roccatello D., Cuadrado M.J. Catastrophic antiphospholipid syndrome (CAPS). Best Pract. Res. Clin. Rheumatol. 2012 Aug. 26(4). 535-41.
- Heegaard N.H., West-Nørager M., Tanassi J.T. et al. Circulating antinuclear antibodies in patients with pelvic masses are associated with malignancy and decreased survival. PLoS One. 2012. 7(2). e30997.
- Fernández-Madrid F., VandeVord P.J., Yang X. et al. Antinuclear antibodies as potential markers of lung cancer. Clin. Cancer Res. 1999 Jun. 5(6). 1393-1400.
- Gökhan Özgür, Cengiz Beyan. Therapeutic apheresis in the treatment of catastrophic antiphospholipid syndrome. Transfusion and Apheresis Science. 2018. 57(1). 13-15.
- Rodriguez-Pintó I., López-Benjume B., Espinosa G., Cervera R. Catastrophic antiphospholipid syndrome. Revista Colombiana de Reumatología. 2021. 28(1). 39-43.
- Ruffatti A., Silvestro G., Marson P. et al. Catastrophic antiphospholipid syndrome: Lessons from 14 cases successfully treated in a single center. J. Autoimmun. 2018. 93. 124-130.

/81.jpg)
/82.jpg)